三己胺 | 102-86-3
中文名称
三己胺
中文别名
三正己胺;三正己基胺;三-N-己基胺
英文名称
tri-n-hexylamine
英文别名
trihexylamine;THA;N,N-dihexylhexan-1-amine
CAS
102-86-3
化学式
C18H39N
mdl
——
分子量
269.514
InChiKey
DIAIBWNEUYXDNL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:150-159 °C/12 mmHg (lit.) 263-265 °C (lit.)
-
密度:0.794 g/mL at 25 °C (lit.)
-
蒸气密度:9.3 (vs air)
-
闪点:>230 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
LogP:4.47 at 25℃ and pH8-9
-
物理描述:Liquid
-
保留指数:1740;1730
-
稳定性/保质期:
避免与强氧化剂和酸接触。使用时应穿戴防护服。
计算性质
-
辛醇/水分配系数(LogP):7.3
-
重原子数:19
-
可旋转键数:15
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:9
-
危险品标志:Xi
-
安全说明:S26,S36,S61
-
危险类别码:R22,R51/53,R34
-
WGK Germany:3
-
海关编码:2921199090
-
包装等级:III
-
危险类别:9
-
危险品运输编号:UN 3082 9/PG 3
-
危险性防范说明:P501,P273,P260,P270,P264,P280,P391,P314,P337+P313,P305+P351+P338,P301+P312+P330
-
危险性描述:H302,H319,H372,H410
-
储存条件:储存于阴凉、通风的库房,远离火种、热源。应与氧化剂、酸性物质等分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| Name: | Trihexylamine 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 102-86-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 102-86-3 | Trihexylamine | 98% | 203-062-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 102-86-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: < 1 mm Hg @ 20 deg
Viscosity: Not available.
Boiling Point: 263 - 265 deg C @ 760.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .7984g/cm3
Molecular Formula: C18H39N
Molecular Weight: 269.51
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 102-86-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Trihexylamine - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: AMINES, LIQUID, CORROSIVE, N.O.S.*
Hazard Class: 8
UN Number: 2735
Packing Group: III
IMO
Shipping Name: AMINES, LIQUID, CORROSIVE, N.O.S.
Hazard Class: 8
UN Number: 2735
Packing Group: III
RID/ADR
Shipping Name: AMINES, LIQUID, CORROSIVE, N.O.S.
Hazard Class: 8
UN Number: 2735
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 102-86-3: No information available.
Canada
CAS# 102-86-3 is listed on Canada's DSL List.
CAS# 102-86-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 102-86-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
用作溶剂和有机合成中间体。
用途简介
暂无具体介绍。
用途
用作溶剂和有机合成中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基己胺 N,N-dimethylhexylamine 4385-04-0 C8H19N 129.246 二已胺 dihexylamine 143-16-8 C12H27N 185.353 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二已胺 dihexylamine 143-16-8 C12H27N 185.353 N,N-二己基甲酰胺 N,N-di-1-hexylformamide 14287-94-6 C13H27NO 213.363
反应信息
-
作为反应物:参考文献:名称:胺和酰胺的氨解转移脱氢:一种将氮化合物转化为腈的通用方法摘要:胺脱氢已被认为是腈合成的有效方法。目前,这种方法仅限于伯胺的(氧化)脱氢,通常使用专门的均相催化剂。在这项工作中,使用简单且廉价的烯烃(例如乙烯或丙烯)作为氢清除剂,将胺脱氢转移为腈。范围扩大到仲胺、叔胺甚至醛。额外的氮气由NH 3内置。通过将其与仲酰胺的氨解耦合,证明了该过程的多功能性。这使我们能够将长链聚酰胺 (LCPA) 回收成单体化合物,即α,ω-酰胺腈和二腈。反应使用可回收的非均相 Pt 催化剂在 200 °C 下进行,并添加有限的 NH 3和乙烯。相应的腈的收率高达94%。DOI:10.1039/d3sc02436a
-
作为产物:描述:已腈 在 二(氰基苯)二氯化钯 、 水 、 氢气 、 silica gel 、 2,2'-biquinoline-4,4'-dicarboxylic acid dipotassium salt 作用下, 以 正己烷 为溶剂, 100.0 ℃ 、2.76 MPa 条件下, 反应 24.0h, 生成 三己胺参考文献:名称:六方介孔二氧化硅负载的水相催化剂将腈化学选择性加氢为仲胺或叔胺摘要:揭示了第一种用于腈氢化的负载型水相催化剂。由Pd(PhCN)2 Cl 2,水溶性配体2,2'-联喹啉-4,4'-二羧酸二钾盐和中孔二氧化硅制备的催化剂是一种高效的催化剂,可用于从中选择性地形成仲胺或叔胺芳族或脂族腈。该催化体系是稳定的,可以循环使用三次,而不会损失活性和选择性。此外,这种环境友好的方法是许多均相和非均相催化剂的有吸引力的替代方法,因为它易于制备并且在中等活性条件下具有很高的活性。DOI:10.1002/aoc.4547
-
作为试剂:描述:参考文献:名称:PROCESS FOR PREPARING FORMIC ACID摘要:在三级胺(I)和催化剂的存在下,通过在0.2到30兆帕绝对压力和20到200摄氏度的温度下,将二氧化碳加氢制备甲酸的方法,其中催化剂是包含金的非均相催化剂。公开号:US20130006015A1
文献信息
-
USE OF NITROGEN COMPOUNDS QUATERNISED WITH ALKYLENE OXIDE AND HYDROCARBYL-SUBSTITUTED POLYCARBOXYLIC ACID AS ADDITIVES IN FUELS AND LUBRICANTS申请人:BASF SE公开号:US20160130514A1公开(公告)日:2016-05-12The invention relates to the use of quaternized nitrogen compounds as a fuel and lubricant additive or kerosene additive, such as in particular as a detergent additive, for decreasing or preventing deposits in the injection systems of direct-injection diesel engines, in particular in common rail injection systems, for decreasing the fuel consumption of direct-injection diesel engines, in particular of diesel engines having common rail injection systems, and for minimizing the power loss in direct-injection diesel engines, in particular in diesel engines having common rail injection systems; the invention further relates to the use as an additive for petrol, in particular for operation of DISI engines.该发明涉及将季铵化氮化合物用作燃料和润滑剂添加剂或煤油添加剂,特别是作为清洁剂添加剂,用于减少或预防直喷柴油发动机的喷射系统中的沉积物,在特定是在共轨喷射系统中,用于降低直喷柴油发动机的燃料消耗,特别是具有共轨喷射系统的柴油发动机,并用于减少直喷柴油发动机的功率损失,特别是在具有共轨喷射系统的柴油发动机中;该发明还涉及将其用作汽油添加剂,特别是用于DISI发动机的运行。
-
One-Pot Synthesis of Tertiary Amides from Organic Trichlorides through Oxygen Atom Incorporation from Air by Convergent Paired Electrolysis作者:Zhongli Luo、Kenji Imamura、Yoshihito Shiota、Kazunari Yoshizawa、Yoshio Hisaeda、Hisashi ShimakoshiDOI:10.1021/acs.joc.1c00161日期:2021.4.16A convergent paired electrolysis catalyzed by a B12 complex for the one-pot synthesis of a tertiary amide from organic trichlorides (R-CCl3) has been developed. Various readily available organic trichlorides, such as benzotrichloride and its derivatives, chloroform, dichlorodiphenyltrichloroethane (DDT), trichloro-2,2,2-trifluoroethane (CFC-113a), and trichloroacetonitrile (CNCCl3), were converted
-
一种含烷基和芳基嘧啶类化合物的合成方法申请人:新乡医学院公开号:CN111362880B公开(公告)日:2023-01-13
-
Transition-Metal-Free C–N Bond Activation: Synthesis of α,α-Bis(arylthio) Aldehydes作者:Chen-Liang Deng、Xu-Hong Gao、Wei Xu、Xing-Guo ZhangDOI:10.1055/s-0036-1588135日期:——bond activation of tertiary amines and subsequent aryl thiolation has been explored. A variety of disulfides and series of tertiary amines were efficiently converted into the corresponding α,α-bis(arylthio) aldehydes in moderate to good yields. The reaction proceeded well under transition-metal-free conditions via a C–N activation process.
-
Iodine Mediated Addition of Trialkylamines to Dimethyl Acetylenedicarboxylate作者:Quanping Wu、Hui-Fang Liu、Yue Zhang、Shiyu Shen、Song XueDOI:10.2174/15701786113109990027日期:2013.9.1Addition reaction of tertiary amines to dimethyl acetylenedicarboxylate mediated by molecular iodine is described. The trialkylamines afford dealkylative adducts in moderate to good yields. Amines containing an aryl group react sluggishly in low yields with a loss of alkyl group.
表征谱图
-
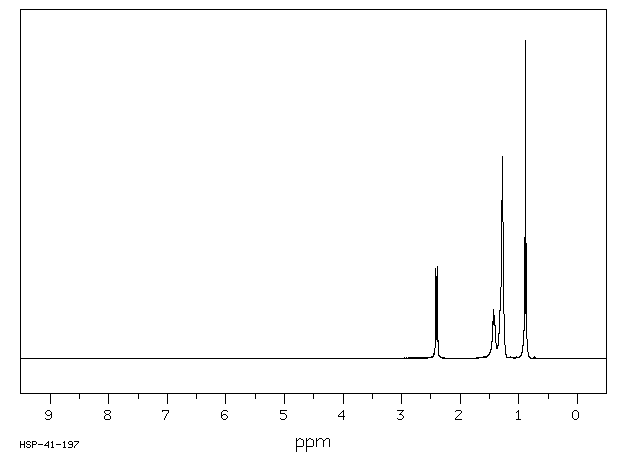
氢谱1HNMR
-
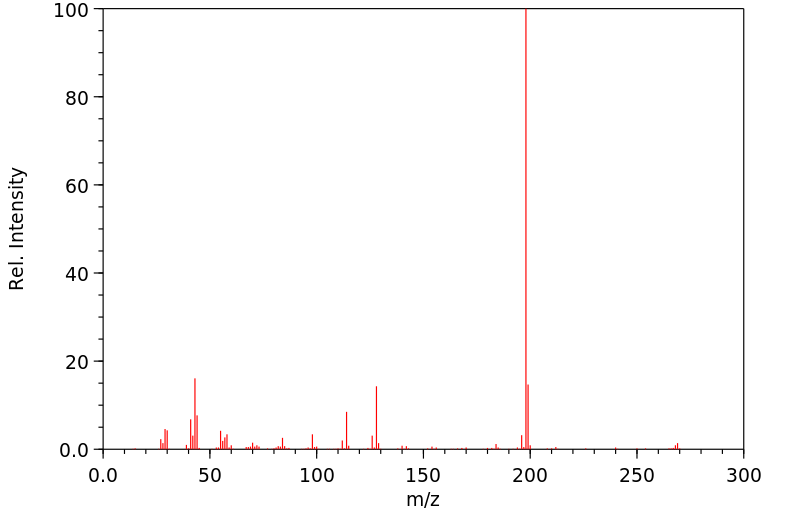
质谱MS
-
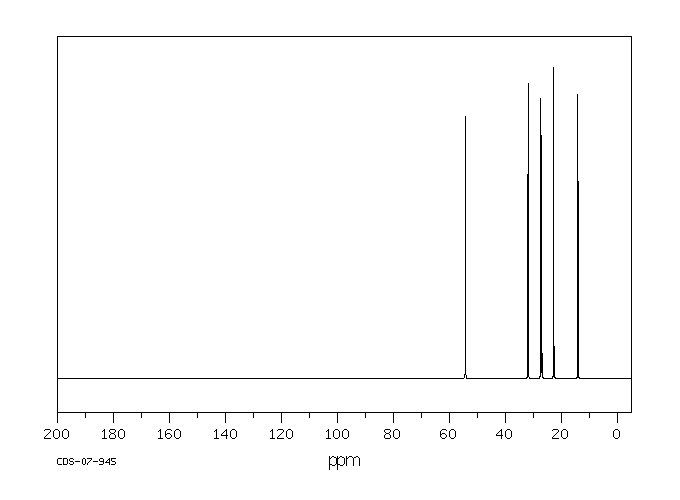
碳谱13CNMR
-
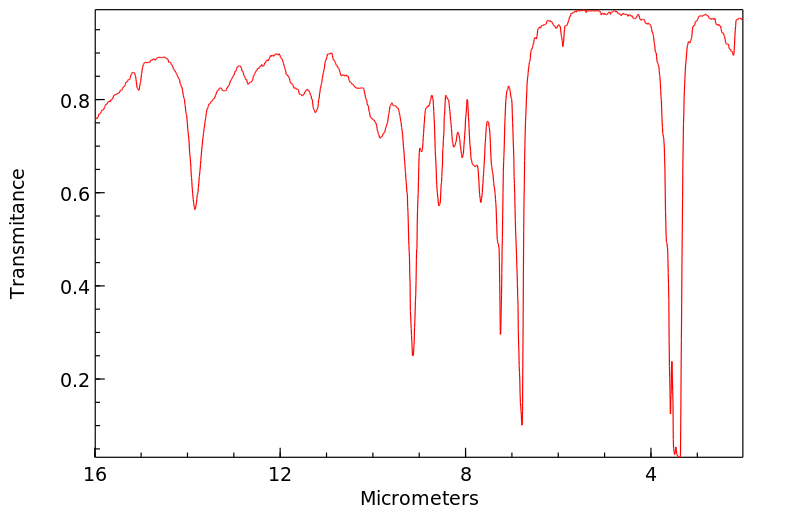
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷