丙氧基苯 | 622-85-5
中文名称
丙氧基苯
中文别名
丙氧苯;苯丙醚
英文名称
propoxybenzene
英文别名
phenyl propyl ether;propyloxybenzene;n-propoxybenzene;3-phenoxy propane
CAS
622-85-5
化学式
C9H12O
mdl
MFCD00039935
分子量
136.194
InChiKey
DSNYFFJTZPIKFZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-28°C(lit.)
-
沸点:190°C(lit.)
-
密度:0.9474
-
最大波长(λmax):279nm(EtOH)(lit.)
-
保留指数:1064.7;1078;1089
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2909309090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Phenyl propyl ether
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Phenyl propyl ether
CAS number: 622-85-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H12O
Molecular weight: 136.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Phenyl propyl ether
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Phenyl propyl ether
CAS number: 622-85-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H12O
Molecular weight: 136.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-苯氧基-1-丙醇 3-phenoxypropanol 6180-61-6 C9H12O2 152.193 3-苯氧基溴丙烷 3-bromophenoxypropane 588-63-6 C9H11BrO 215.09 —— 3-phenoxypropionaldehyde 22409-86-5 C9H10O2 150.177 苯基炔丙基醚 1-phenoxy-2-propyne 13610-02-1 C9H8O 132.162 烯丙基苯基醚 allyl phenyl ether 1746-13-0 C9H10O 134.178 (3-苯氧基丙基)硼酸 (3-phenoxypropyl)boronic acid 90535-47-0 C9H13BO3 180.011 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-丙氧基苯酚 4-propoxyphenol 18979-50-5 C9H12O2 152.193 1-氯-4-丙氧基苯 1-chloro-4-propoxybenzene 33382-58-0 C9H11ClO 170.639 —— p-Propoxythiophenol 1126-83-6 C9H12OS 168.26 4-n-丙氧基溴苯 1-bromo-4-propoxybenzene 39969-56-7 C9H11BrO 215.09 苯甲醚 methoxybenzene 100-66-3 C7H8O 108.14 4-丙氧基苯甲醛 4-propoxybenzaldehyde 5736-85-6 C10H12O2 164.204 1-(氯甲基)-4-丙氧基苯 (4-chloromethyl-phenyl)-propyl ether 40141-11-5 C10H13ClO 184.666 1-氯-2-丙氧基苯 o-chloropropoxybenzene 33382-57-9 C9H11ClO 170.639 —— n-Propyl 4-n-propylphenyl ether 74663-46-0 C12H18O 178.274 —— 4-propoxycumene 28530-35-0 C12H18O 178.274 —— (4-benzyl-phenyl)-propyl ether 91404-31-8 C16H18O 226.318 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Schuikin et al., Vestnik Moskovskogo Universiteta, 1957, vol. 12, # 5, p. 125,129摘要:DOI:
-
作为产物:参考文献:名称:用于受阻烯烃加氢的二齿NHC-钴催化剂摘要:在此,我们报告了一系列易于获得的双齿N-杂环卡宾(NHC)钴催化剂,该催化剂能够在温和条件下氢化受阻烯烃。通过X射线衍射,元素分析,ESI-HRMS和磁矩测量对四配位的双齿NHC-Co(II)配合物进行表征,揭示了扭曲的四面体几何形状和金属中心的高自旋构型。从容易获得的NHC前体CoCl 2和NaHBEt 3获得的原位形成的催化体系的活性与明确定义的NHC-钴催化剂的活性相同。这突出了该反应系统的潜在用途。DOI:10.1021/acs.organomet.0c00498
文献信息
-
BRM TARGETING COMPOUNDS AND ASSOCIATED METHODS OF USE申请人:Arvinas Operations, Inc.公开号:US20190300521A1公开(公告)日:2019-10-03The present disclosure relates to bifunctional compounds, which find utility as modulators of SMARCA2 or BRM (target protein). In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a ligand that binds to the Von Hippel-Lindau E3 ubiquitin ligase, and on the other end a moiety which binds the target protein, such that the target protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of target protein. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of target protein. Diseases or disorders that result from aggregation or accumulation of the target protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为SMARCA2或BRM(靶蛋白)的调节剂具有实用性。具体而言,本公开涉及包含一端结合Von Hippel-Lindau E3泛素连接酶的配体,另一端结合靶蛋白的双功能化合物,使得靶蛋白与泛素连接酶靠近以实现靶蛋白的降解(和抑制)。本公开展示了与靶蛋白降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由靶蛋白聚集或积累导致的疾病或紊乱。
-
Ambient Hydrogenation and Deuteration of Alkenes Using a Nanostructured Ni‐Core–Shell Catalyst作者:Jie Gao、Rui Ma、Lu Feng、Yuefeng Liu、Ralf Jackstell、Rajenahally V. Jagadeesh、Matthias BellerDOI:10.1002/anie.202105492日期:2021.8.16selective hydrogenation and deuteration of a variety of alkenes is presented. Key to success for these reactions is the use of a specific nickel-graphitic shell-based core–shell-structured catalyst, which is conveniently prepared by impregnation and subsequent calcination of nickel nitrate on carbon at 450 °C under argon. Applying this nanostructured catalyst, both terminal and internal alkenes, which
-
PHOTOPROTECTIVE COMPOSITIONS COMPRISING PHOTOSENSITIVE 1,3,5-TRIAZINE COMPOUNDS, DIBENZOYLMETHANE COMPOUNDS AND SILICEOUS S-TRIAZINES SUBSTITUTED WITH TWO AMINOBENZOATE OR AMINOBENZAMIDE GROUPS申请人:L'OREAL公开号:US20170135933A1公开(公告)日:2017-05-18UV-photoprotective, topically applicable cosmetic/dermatological compositions contain: (a) at least one dibenzoylmethane compound, (b) at least one 1,3,5-triazine compound that is photosensitive in the presence of a dibenzoylmethane compound, and (c) at least one siliceous s-triazine compound substituted with two aminobenzoate or aminobenzamide groups, or a tautomeric form thereof, the 1,3,5-triazine compounds being improvedly photostable in such compositions.
-
[EN] NRTI THERAPIES<br/>[FR] THÉRAPIES NRTI申请人:UNIV LIVERPOOL公开号:WO2020128525A1公开(公告)日:2020-06-25Polymer-of-prodrug (POP) materials enable new nucleoside reverse transcriptase inhibitor (NRTI) therapy strategies. The materials are prodrugs of NRTIs in the form of polymers. Suitable materials include products which are polymeric NRTI delivery systems comprising polymeric materials which are capable of degradation after administration to release NRTIs or NRTI prodrugs which themselves are capable of metabolism to the parent NRTIs. The NRTIs may optionally be selected from tenofovir (TFV), emtricitabine (FTC), lamivudine (3TC) and MK-8591 (EFdA). The invention facilitates long-acting (LA) regimens. Constructs of the materials may be in the form of injectable compositions or implants.
-
Sodium borohydride-nickel chloride hexahydrate in EtOH/PEG-400 as an efficient and recyclable catalytic system for the reduction of alkenes作者:Kaoxue Li、Chuanchao Liu、Kang Wang、Yang Ren、Fahui LiDOI:10.1039/c8ra00905h日期:——An efficient, safe and one-pot convenient catalytic system has been developed for the reduction of alkenes using NaBH4–NiCl2·6H2O in EtOH/PEG-400 under mild conditions. In this catalytic system, a variety of alkenes (including trisubstituted alkene α-pinene) were well reduced and the Ni catalyst could be recycled.
表征谱图
-
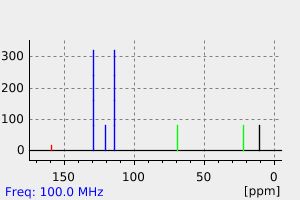
氢谱1HNMR
-
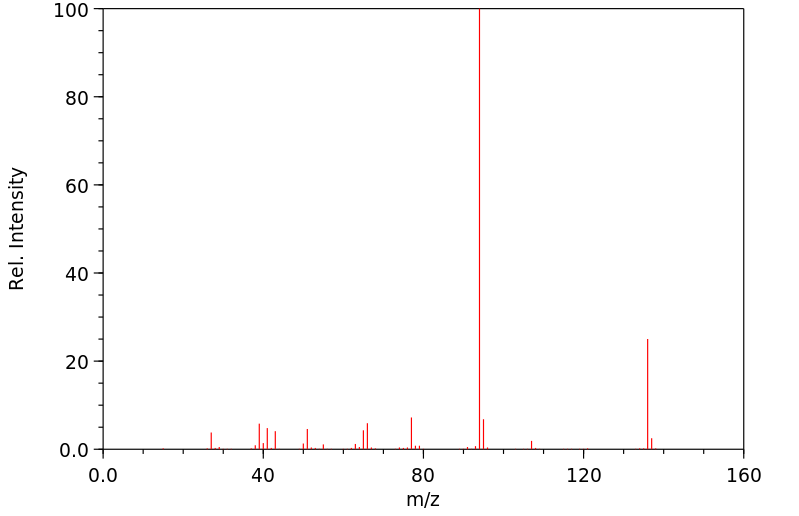
质谱MS
-
碳谱13CNMR
-
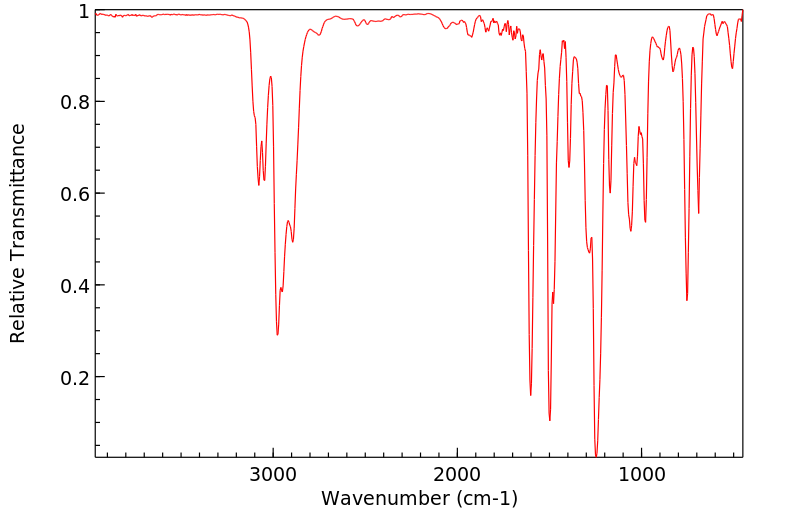
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯