[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON
申请人:BIOCAD JOINT STOCK CO
公开号:WO2018092047A1
公开(公告)日:2018-05-24
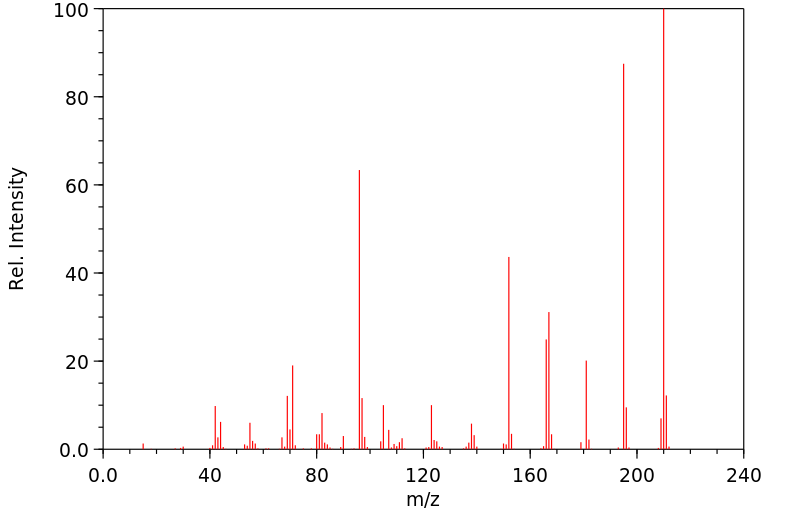
The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.
本发明涉及一种新的化合物,其
化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为
CH2,NH,O或
化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[
化学式II]每个R7,R8,R9,R10独立地为
乙烯基,甲基
乙炔基;Hal为CI,Br,I,F,具有布鲁顿
酪氨酸激酶(Btk)
抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。