adamantan-1-ol | 768-95-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:247 °C (subl.) (lit.)
-
沸点:214.73°C (rough estimate)
-
密度:0.8544 (rough estimate)
-
闪点:240°C
-
溶解度:氯仿(微溶)、甲醇(微溶)
计算性质
-
辛醇/水分配系数(LogP):1.57
-
重原子数:11.0
-
可旋转键数:0.0
-
环数:3.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.23
-
氢给体数:1.0
-
氢受体数:1.0
安全信息
-
危险品标志:Xi
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29061900
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:AU4980000
-
包装等级:III
-
危险类别:4.1
-
危险性防范说明:P240,P210,P264,P280,P370+P378,P337+P313
-
危险性描述:H315,H319,H335,H228
SDS
制备方法与用途
1-金刚烷醇(英文名:1-Adamantanol),又称1-氢氧基金刚烷、1-三环[3.3.1.1(3,7)]癸醇,常温下为白色结晶粉末,熔点超过240℃。它溶于有机溶剂而不溶于水,并具有升华性。主要用途包括制造合成金刚烷衍生物和阿达巴林;与偏二氯乙烯反应可得1-金刚烷乙酸,后者再经过溴化、水解为3-羟基-1-金刚烷乙酸,进而与偏二氯乙烯反应得到1,3-金刚烷二乙酸。
此外,在98%硫酸介质中室温下,1-金刚烷醇可发生傅克烷基化反应直接合成1,3-二(4-乙酰氨基苯基)金刚烷,通过碱水解可进一步转化为1,3-二(4-苯胺)金刚烷。这种合成路线具有产率高、易提纯等优点,极大地便利了1,3-金刚烷二芳基衍生物的合成。
用途1-金刚烷醇主要用于合成金刚烷类化合物,并作为制造合成金刚烷衍生物和阿达巴林的关键中间体;此外,它还与偏二氯乙烯反应生成1-金刚烷乙酸。
反应信息
-
作为反应物:描述:adamantan-1-ol 在 邻苯二甲酰过氧化物 、 四丁基溴化铵 作用下, 以 1,2-二氯乙烷 为溶剂, 反应 3.0h, 以33%的产率得到endo-7-(bromomethyl)bicyclo[3.3.1]nonan-3-one参考文献:名称:布朗斯台德碱式束缚的酰氧基自由基诱导的氢原子转移-电子转移使环烷醇的可见光增强开环摘要:提出了结合了PPO / TBAX氧化剂系统和蓝色LED辐射的无金属环烷醇开环/卤化反应。该方法在温和条件下以中等至极佳的收率生产出多种γ,δ甚至更遥远的卤代酮。有趣的是,实验和计算研究表明,布朗斯台德碱式束缚的酰氧基基团诱导了新颖的环尺寸依赖性的一致/逐步(四元/五元至五元至八元环)氢原子转移-电子转移,这表明氢氧自由基带来了明显的优势。二酰基过氧化物的环状结构。DOI:10.1021/acs.orglett.8b00161
-
作为产物:描述:α,α-dicyclopropylcyclopropanemethanol 、 1-chloroadamantane 生成 tricyclopropylchloromethane 、 adamantan-1-ol参考文献:名称:Abboud, Jose-Luis M.; Alkorta, Ibon; Davalos, Juan Z., Journal of Organic Chemistry, 2003, vol. 68, # 10, p. 3786 - 3796摘要:DOI:
文献信息
-
<i>N</i>-Bromosuccinimide (NBS) — Selective and effective catalyst for trimethylsilylation of alcohols and phenols using hexamethyldisilazane and their regeneration under mild and neutral reaction conditions作者:Ardeshir Khazaei、Amin Rostami、Ayeh Raiatzadeh、Marjan MahboubifarDOI:10.1139/v07-029日期:2007.5.1
Structurally diverse alcohols and phenols were trimethylsilylated in a clean and efficient reaction with hexamethyldisilazane (HMDS) based on the use of a catalytic amount of N-bromosuccinimide under both dichloromethane and solvent-free conditions at room temperature. Deprotection of trimethylsilyl ethers was also be achieved efficiently in the presence of a catalytic amount of NBS in methanol at ambient temperature.Key words: N-bromosuccinimide, solvent-free, alcohols, phenols, hexamethyldisilazane, trimethylsilyl ether, catalyst, detrimethylsilylation.
-
Palladium-catalyzed silylation of alcohols with hexamethyldisilane作者:Eiji Shirakawa、Koji Hironaka、Hidehito Otsuka、Tamio HayashiDOI:10.1039/b608958e日期:——The combination of hexamethyldisilane and a catalytic amount of [PdCl(η3-C3H5)]2–PPh3 was found to be effective for the trimethylsilylation of alcohols, where both of the two trimethylsilyl groups of hexamethyldisilane were transferred to alcohols without coproduction of any stoichiometric amount of byproduct but H2.
-
一种氟化试剂及脱氧氟化方法
-
Collisionally-induced dissociation mass spectra of organic sulfate anions作者:Athula B. Attygalle、Silvina García-Rubio、Jennifer Ta、Jerrold MeinwaldDOI:10.1039/b009019k日期:——The collisionally-induced dissociation mass spectra of a variety of organic sulfate ester anions are described and mechanistically rationalized. A cyclic syn-elimination pathway, analogous to that of the Cope elimination, is postulated for the commonly observed formation of bisulfate anion (HSO4−, m/z 97). Deuterium labeling experiments confirm that the proton transferred to oxygen during bisulfate碰撞诱导的解离 质谱 各种 有机硫酸盐 酯对阴离子进行了描述并进行了机械合理化。甲环状顺式β-消除途径,类似于该上砂消除,假定为通常观察到的形成硫酸氢盐阴离子(HSO 4 - ,M / Z 97)。氘贴标 实验证实 质子 硫酸氢根消除过程中转移到氧气上的氧通常来自C-2位置,尽管检查了多官能团的光谱 类固醇 揭示了 质子摘要也可能源自更远的站点。精金苯基和乙烯基硫酸根阴离子,其不容易借给自己的环状顺式β-消除,不产生一个M / Z 97离子。相反,这些硫酸盐进行杂溶和均溶的S-O键裂解产生m / z M-80阴离子,这是由于中性SO 3的损失以及m / z 80处的离子(分别对应于SO 3 - loss)引起的。硫酸盐 可以通过均相C–O键裂变产生共振稳定的自由基,例如 苄基可以通过形成m / z 96(SO 4 - ˙)离子来识别硫酸根草酯和硫酸芳樟基酯。
-
Extended Applications of Di-tert-butylsilylene-Directed α-Predominant Galactosylation Compatible with C2-Participating Groups toward the Assembly of Various Glycosides作者:Akihiro Imamura、Akiyoshi Kimura、Hiromune Ando、Hideharu Ishida、Makoto KisoDOI:10.1002/chem.200600832日期:2006.11.24alpha-predominant galactosylation have been extended to the construction of difficult glycan sequences. First, to investigate the compatibility of the alpha-predominant reaction with various glycosylation systems a variety of 4,6-O-DTBS-tethered galactosaminyl or galactosyl donors were synthesized efficiently, which have C2-participating groups with a wide variety of leaving groups such as alkylsulfenyl二叔丁基亚甲硅烷基(DTBS)指导的α占主导地位的半乳糖基化的高通用性已扩展到困难的聚糖序列的构建。首先,为研究α-优势反应与各种糖基化系统的相容性,有效合成了各种4,6-O-DTBS系链的半乳糖胺基或半乳糖基供体,这些供体具有C2参与基团,具有多个离去基团,例如作为烷基亚磺酰基,卤化物,三氯乙酰亚胺基。使用糖基供体的糖基化反应的详细检查结果表明,由4,6-DTBS定向的α-半乳糖基化范围很广。在下一步中,通过采用DTBS定向的糖基化来检查alpha-GalN-Ser / Thr序列的立体选择性构建。因此,各种类型的丝氨酸和苏氨酸衍生物被糖基化,进行了高选择性的α-GalN-Ser/ Thr序列糖基化。此外,DTBS定向的半乳糖基化已成功地用于糖蛋白A的α-四糖基-Ser片段的合成。
表征谱图
-
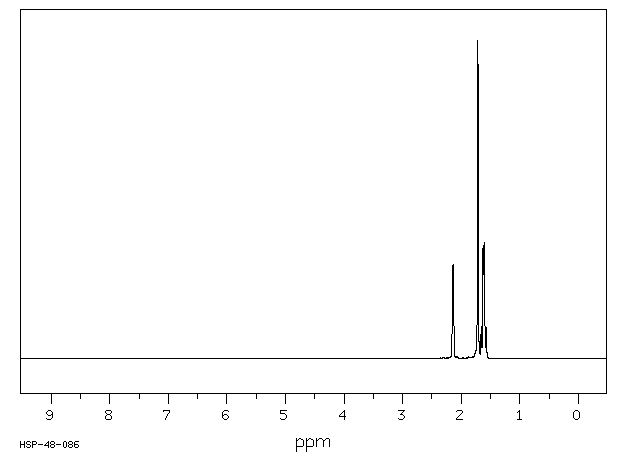
氢谱1HNMR
-
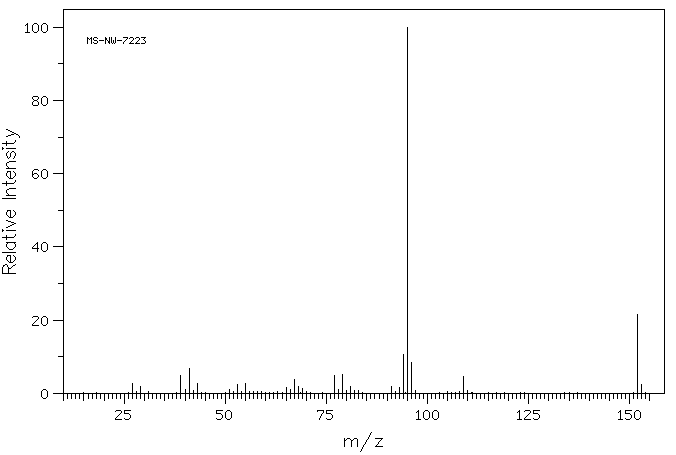
质谱MS
-
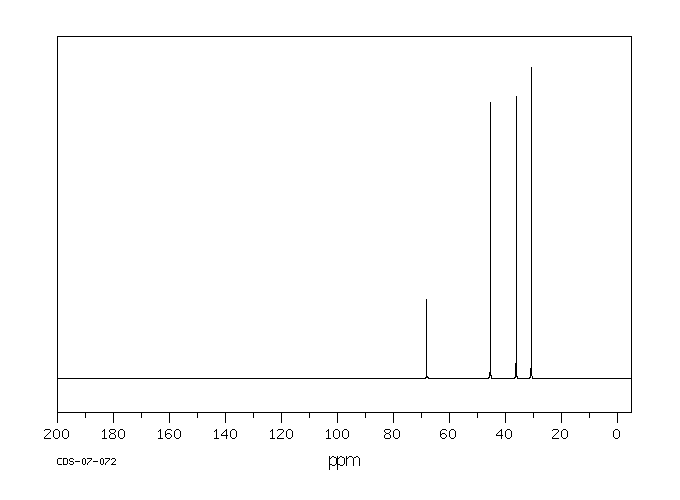
碳谱13CNMR
-
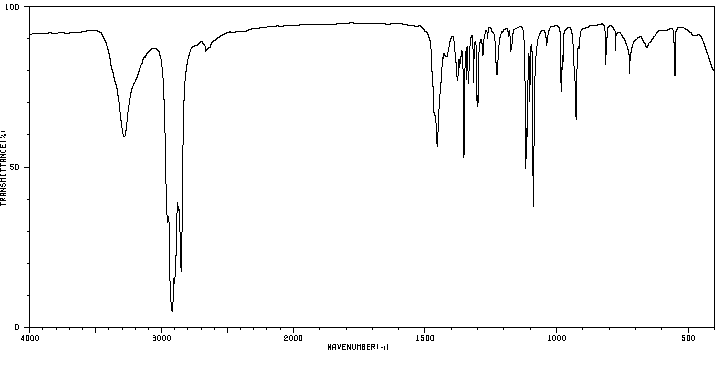
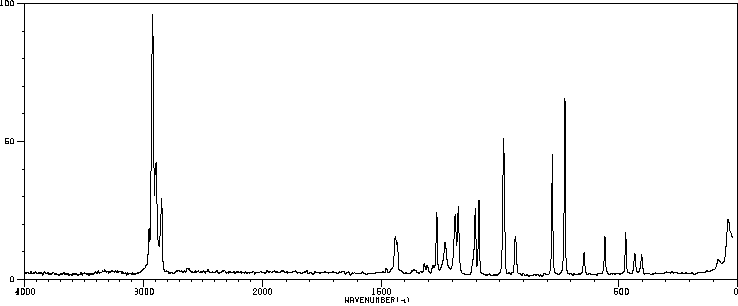
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息