反-4-壬烯 | 10405-85-3
中文名称
反-4-壬烯
中文别名
4-壬烯;1-丁基-2-丙基乙烯;4-壬烯(顺反共混物)
英文名称
(E)-non-4-ene
英文别名
Non-4-en;Trans-4-NONENE
CAS
10405-85-3
化学式
C9H18
mdl
——
分子量
126.242
InChiKey
KPADFPAILITQBG-VQHVLOKHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:144-146 °C(lit.)
-
密度:0.732 g/mL at 25 °C(lit.)
-
闪点:81 °F
-
LogP:4.883 (est)
-
保留指数:893;894;894;894;894;894;884;894;839;840;893
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.78
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险品标志:Xn
-
安全说明:S16,S62
-
危险类别码:R10
-
海关编码:2901299090
-
危险品运输编号:UN 3295 3
SDS
上下游信息
反应信息
-
作为反应物:描述:反-4-壬烯 在 ruthenium trichloride 、 sodium periodate 作用下, 以 乙腈 为溶剂, 反应 0.01h, 以58%的产率得到(4R,5R)-(+)-4,5-nonanediol参考文献:名称:Shing, Tony K. M.; Tam, Eric K. W.; Tai, Vincent W.-F., Chemistry - A European Journal, 1996, vol. 2, # 1, p. 50 - 57摘要:DOI:
-
作为产物:描述:参考文献:名称:Hydroboration. 86. Convenient conversion of aldehydes and ketones into the corresponding alkenes via hydroboration of their enamines. A remarkably simple synthesis of either (Z)- or (E)-alkenes摘要:Aldehydes and ketones are converted into the corresponding alkenes via hydroboration of their enamines. Hydroboration of aldehyde enamines by 9-borabicyclo[3.3.1]nonane (9-BBN), followed by methanolysis, affords the corresponding terminal alkenes in 75-90% yields. Unsaturated aldehyde enamines produce the corresponding dienes under these conditions. Enamines derived from substituted cyclic ketones and heterocyclic ketones are readily accommodated in this reaction to afford the corresponding alkenes in very good yields. The synthesis of pure (Z)- or (E)-alkenes is readily achieved from the same acyclic ketone enamine by modification of the hydroboration-elimination procedure: (A) hydroboration by 9-BBN followed by methanolysis or (B) hydroboration by borane methyl sulfide (BMS) followed by methanolysis and hydrogen peroxide oxidation. Mechanistic rationale is provided.DOI:10.1021/jo00004a038
文献信息
-
Vacuum flash pyrolysis (VFP) of malonyl peroxides: decarboxylation versus decarbonylation of the intermediary α-lactones作者:Waldemar Adam、Carlos Cadiz、Francois MazenodDOI:10.1016/s0040-4039(01)90274-6日期:1981.1Vacuum Flash Pyrolysis (VFP) at ca. 450–500°C and ca. 0.1–0.3 Torr of the spirocyclic malonyl peroxides (2a,b) affords high yields of allenes (5a,b), while the simple malonyl peroxide (2c) leads to ketone (4), derived respectively from decarboxylation and decarbonylation of the intermediary α-lactones (3).真空闪速热解(VFP)的温度约为 450–500°C左右 螺环过氧化丙二酰(2a,b)的0.1–0.3托可提供高产率的丙二烯(5a,b),而简单的丙二酰过氧化物(2c)则可生成酮(4),其分别源自中间α的脱羧和脱羰-内酯(3)。
-
Homoallylic substitution reactions of lithium dialkyl cuprates with cyclopropylcarbinyl halides: mechanistic considerations作者:Robert T. Hrubiec、Michael B. SmithDOI:10.1016/s0040-4020(01)91792-2日期:1984.1kanes, , react to give good yields of the homoallylic substitution product, . Less reactive organocuprates react with to give mixtures of and the direct substitution product, . These results are consistent with a copper(I) radical intermediate which undergoes facile rearrangement prior to reductive coupling.
-
Double bond geometry of the alkenes produced by oxidative elimination of alkyl phenyl selenides and tellurides作者:Sakae Uemura、Yasuyuki Hirai、Kouichi Ohe、Nobuyuki SugitaDOI:10.1039/c39850001037日期:——Treatment of secondary-alkyl Phenyl selenides with various oxidants affords the corresponding trans-alkene highly selectively irrespective of the amount of oxidant, while in the case of the tellurium, analogues the double bond geometry of the product alkene depends markedly on the amount of oxidant, the trans-isomer being formed highly selectively with 1 equiv. oxidant and the proportion of the cis-isomer
-
High Yield of Liquid Range Olefins Obtained by Converting <i>i</i>-Propanol over Zeolite H-ZSM-5作者:Uffe V. Mentzel、Saravanamurugan Shunmugavel、Sarah L. Hruby、Claus H. Christensen、Martin S. HolmDOI:10.1021/ja907692t日期:2009.11.25Methanol, ethanol, and i-propanol were converted under methanol-to-gasoline (MTH)-like conditions (400 degrees C, 1-20 bar) over zeolite H-ZSM-5. For methanol and ethanol, the catalyst lifetimes and conversion capacities are comparable, but when i-propanol is used as the reactant, the catalyst lifetime is increased dramatically. In fact, the total conversion capacity (calculated as the total amount
-
1,2-Dialkylvinylsilanes from α-silyl epoxides via organolithium reagents作者:Braulio Santiago、Carlos Lopez、John A. SoderquistDOI:10.1016/0040-4039(91)80805-g日期:1991.7The reactions of cis-α-epoxysilanes (1) with an excess of alkyllithium reagents were found to cleanly provide 1,2-dialkylvinylsilanes (3). A model for this reductive alkylation is advanced which explains the role of substituents in determining the product stereochemistry.
表征谱图
-
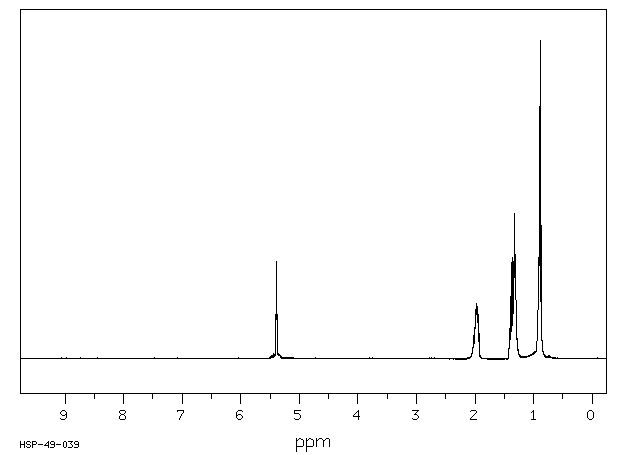
氢谱1HNMR
-
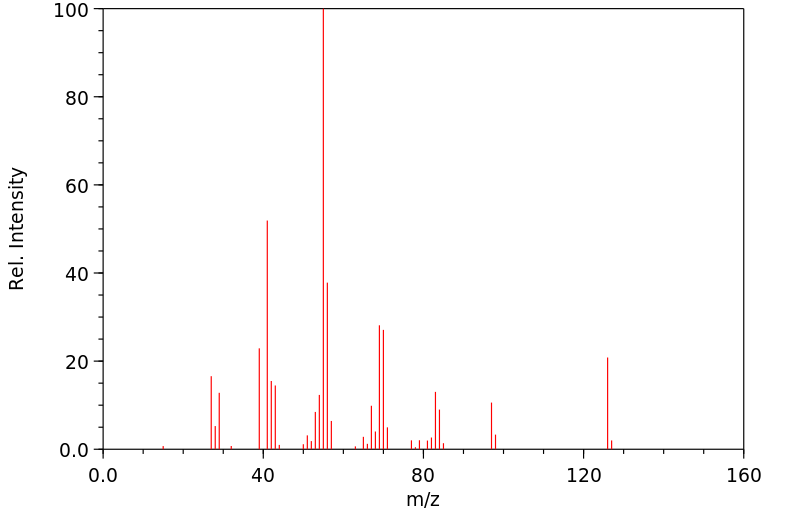
质谱MS
-
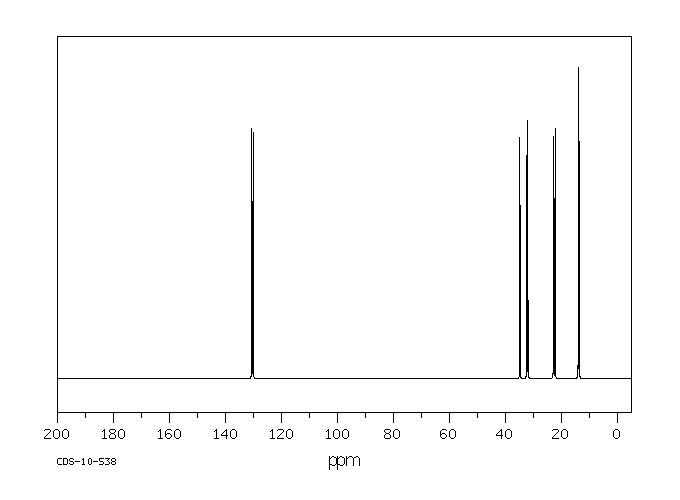
碳谱13CNMR
-
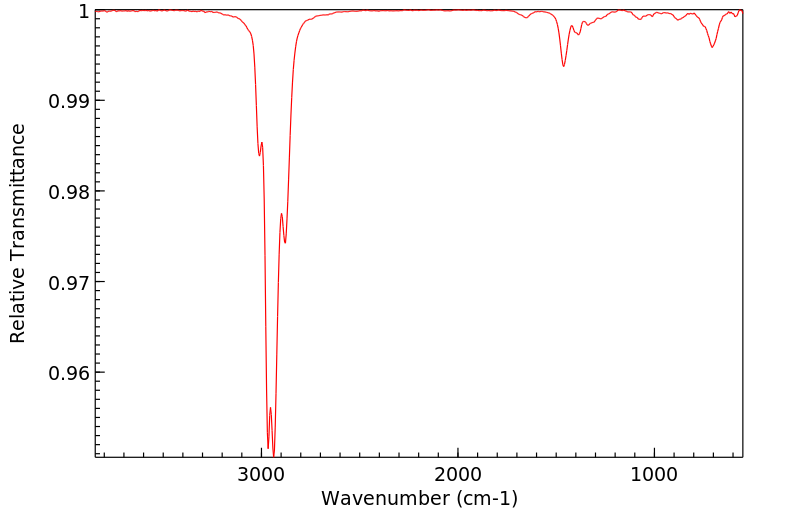
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-