反式-5-十一碳烯 | 764-97-6
中文名称
反式-5-十一碳烯
中文别名
——
英文名称
(E)-undec-5-ene
英文别名
(E)-5-undecene
CAS
764-97-6
化学式
C11H22
mdl
——
分子量
154.296
InChiKey
NGCRXXLKJAAUQQ-PKNBQFBNSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-61.1°C
-
沸点:187.71°C (estimate)
-
密度:0.7497
-
LogP:5.902 (est)
-
保留指数:1090;1082
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:11
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Asinger,F. et al., Chemische Berichte, 1964, vol. 97, p. 1568 - 1575摘要:DOI:
-
作为产物:参考文献:名称:A new route to α-trialkylsilyl aldehydes. The first isolation of α-trimethylsilyl aldehydes摘要:通过溴锂交换和三烷基硅基的 1â3 迁移,从三甲基硅基和三乙基硅基 δ溴烯醇醚 1 中得到δ-三甲基硅基和δ-三乙基硅基醛 6。DOI:10.1039/c39930001763
文献信息
-
A Stereoselective Synthesis of<i>trans</i>-1,2-Disubstituted Alkenes Based on the Condensation of Aldehydes with Metallated 1-Phenyl-1<i>H</i>-tetrazol-5-yl Sulfones作者:Paul R. Blakemore、William J. Cole、Philip J. Kocieński、Andrew MorleyDOI:10.1055/s-1998-1570日期:1998.1The reaction of metallated 1-phenyl-1H-tetrazol-5-yl sulfones and aldehydes gives good yields and stereoselectivity of trans-1,2-disubstituted alkenes when potassium or sodium hexamethyldisilazide is used as base and 1,2-dimethoxyethane is used as solvent.
-
m-C2B10H11HgCl/AgOTf-Catalyzed Reaction for Reductive Deoxygenation作者:Naoto Yamasaki、Marina Kanno、Kyohei Sakamoto、Yusuke Kasai、Hiroshi Imagawa、Hirofumi YamamotoDOI:10.1055/s-0036-1588554日期:2018.1A m -C 2 B 10 H 11 HgCl/AgOTf-catalyzed reaction of allyl silyl ethers with N -Boc- N ′-tosylhydrazine has been developed. Under mild conditions, the resulting allyl hydrazine products were transformed into naked alkenes in good yield. Furthermore, the used m -C 2 B 10 H 11 HgCl could be recovered quantitatively.
-
Decarbonylative Olefination of Aldehydes to Alkenes作者:Diana Ainembabazi、Christopher Reid、Amanda Chen、Nan An、Jakub Kostal、Adelina Voutchkova-KostalDOI:10.1021/jacs.9b12354日期:2020.1.15alternatives to Wittig chem-istry are needed to construct olefins from carbonyl compounds, but none have been developed to-date. Here we report an atom-economical olefination of carbonyls via aldol-decarbonylative coupling of aldehydes using robust and recyclable supported Pd catalysts, producing only CO and H2O as waste. The reaction affords homocoupling of aliphatic al-dehydes, as well as heterocoupling
-
A Novel Synthesis of Internal Alkenyldialkylborane by the Reaction of 1-Halo-1-alkenyldialkylborane with Grignard Reagent作者:Akira Arase、Masayuki Hoshi、Yuzuru MasudaDOI:10.1246/bcsj.57.209日期:1984.1To synthesize internal alkenyldialkylboranes, coupling reactions were carried out by using 1-halo-l-alkenyldialkylboranes and Grignard reagents. Hydrogen peroxide oxidation and protonolysis with acetic acid of the reaction product revealed that internal (E)-alkenyldialkylborane was formed in 60–90% yield.
-
Regioselective Hydrogenation Using Platinum-Support Zeolite Modified by CVD-Method作者:Hideyuki Kuno、Makoto Shibagaki、Kyoko Takahashi、Ichiro Honda、Hajime MatsushitaDOI:10.1246/bcsj.64.2508日期:1991.8A platinum-support zeolite coupled with a silica layer was prepared by Chemical Vapor Deposition (CVD) of tetraethoxysilane, and was investigated in order to determine the catalytic characterization in an analysis of the surface. With this catalyst system, it was demonstrated that the terminal carbon–carbon double bond is preferentially hydrogenated in the case of several unsaturated hydrocarbons. Further, it was elucidated that a highly regioselective hydrogenation of compounds which possess plural double bonds was achieved over this catalyst system. As a result, a reaction model regarding this catalyst system has been put forward.
表征谱图
-
氢谱1HNMR
-
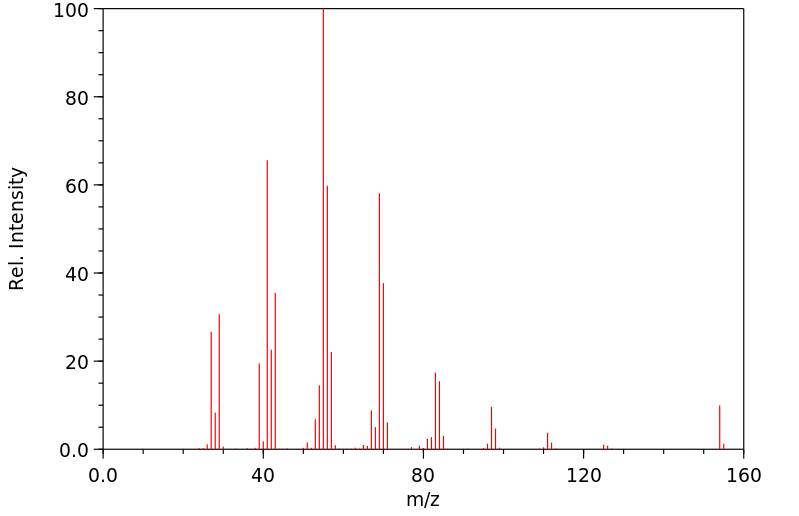
质谱MS
-
碳谱13CNMR
-
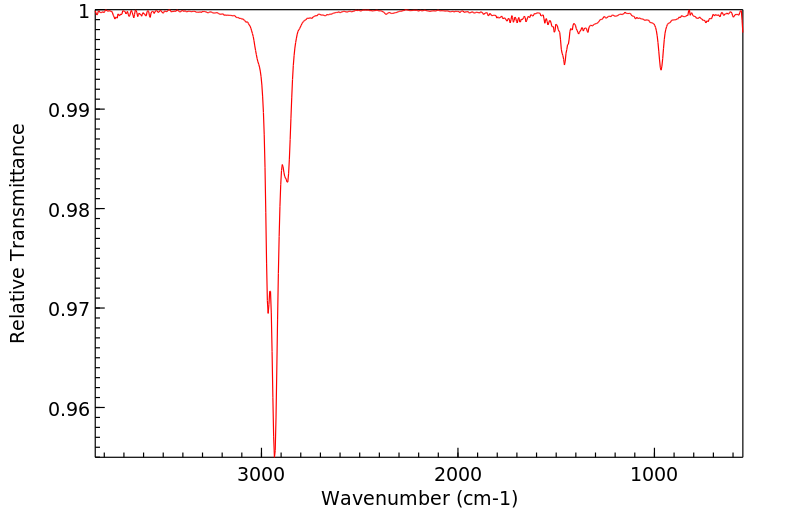
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-