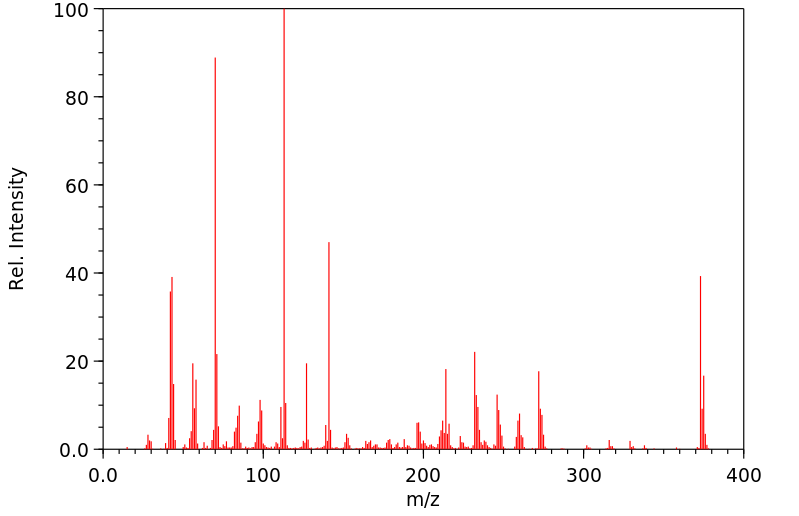
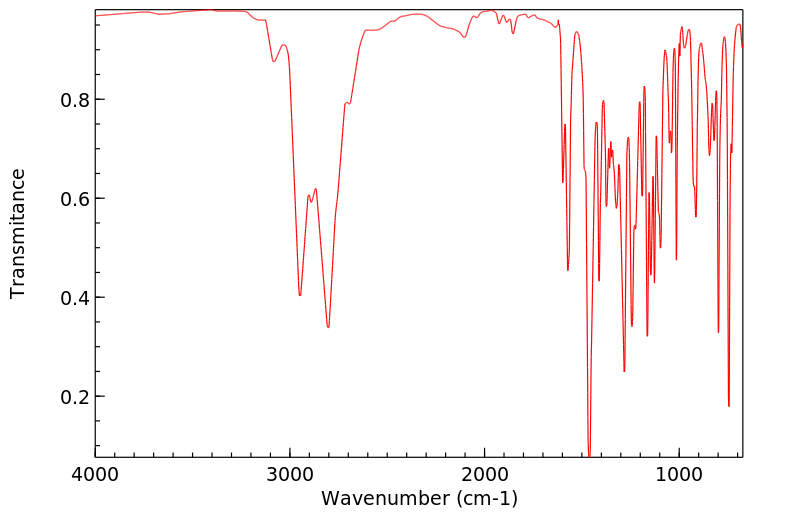
代谢
氯丙嗪经过肝脏代谢,涉及氧化、羟基化、脱甲基、亚砜形成以及与
葡萄糖醛酸的结合。氧化反应由CYP2D6介导。口服和颊部给药后在血浆中检测到了N-去甲基
氯丙嗪,以及
氯丙嗪亚砜、
氯丙嗪7-羟基和
氯丙嗪亚砜4'-N-氧化物,
氯丙嗪可能进入肠肝循环。
来源:DrugBank
代谢
大多数
吩噻嗪类药物的代谢物在药理上是无效的;然而,某些代谢物(例如,
7-羟基氯丙嗪,美索利嗪)显示出适度的药理活性,并可能对药物的作用有所贡献。有限的证据表明,一些
吩噻嗪类药物(例如,
氯丙嗪)可能会诱导其自身的代谢。/
吩噻嗪类药物一般声明/
来源:Hazardous Substances Data Bank (HSDB)
代谢
主要在肝脏通过氧化、羟基化、脱甲基、亚砜形成和与
葡萄糖醛酸结合进行代谢;侧链的代谢改变也可能发生。
来源:Hazardous Substances Data Bank (HSDB)
代谢
经过对大鼠长期给予
哌嗪取代的
吩噻嗪类药物后,组织中含有了药物代谢物,其中
哌嗪环通过多次氧化N-脱烷基化反应断裂,生成了取代的
乙二胺。因此,从
氯丙嗪中得到了N-[γ-(
2-氯吩噻嗪基-10)-丙基]
乙二胺...
来源:Hazardous Substances Data Bank (HSDB)
代谢
产生产氧-2-
氯-10-(3-(4-甲基
哌嗪-1-基)丙基)
吩噻嗪和2-
氯-10-(3-(4-甲基
哌嗪-1-基)丙基)
吩噻嗪亚砜的大鼠
来源:Hazardous Substances Data Bank (HSDB)
毒理性
氯丙嗪的作用机制尚未完全确定,但可能主要与其抗
多巴胺能效应有关。
氯丙嗪阻断了D2体的树突自身受体,导致中脑边缘系统突触后
多巴胺受体的阻断和
多巴胺周转率的增加。
氯丙嗪还具有抗吐效果,这可以归因于在
化学感受器触发区的
多巴胺阻断。
氯丙嗪还阻断了抗
胆碱能和α-
肾上腺素能受体,其中α(1)-
肾上腺素能受体的阻断导致镇静、肌肉松弛和低血压。
来源:Toxin and Toxin Target Database (T3DB)
毒理性
在
氯丙嗪治疗期间,肝脏测试异常并不常见,这可能是因为它很少长期给予或慢性高剂量使用。在治疗期间可能会出现
氨基转移酶升高,但它们通常是轻微的、无症状的、短暂的,并且在继续用药的情况下也是可逆的。已经报道过由于
氯丙嗪引起的临床上明显的急性肝损伤的罕见病例,这些病例类似于与
氯丙嗪相关的胆汁淤积性肝损伤。黄疸的出现通常在1到4周内,血清酶升高的模式通常是胆汁淤积性或混合型。在某些病例中会出现免疫过敏特征(发热和嗜酸性粒细胞增多),但它们通常是轻微的和自限性的;自身
抗体罕见。肝脏活检通常显示胆汁淤积性肝炎。重要的是,
氯丙嗪引起的黄疸可能会延长,并且已经与罕见的消失胆管综合征(案例1)相关联,这可能是致命的,或者最终需要肝移植。
来源:LiverTox
毒理性
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
药物性肝损伤标注:低药物性肝损伤关注
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
毒理性
严重程度等级:2
来源:Drug Induced Liver Injury Rank (DILIrank) Dataset
吸收、分配和排泄
口服给药后,据报道,
氯丙嗪能很好地从胃肠道吸收。药理作用的出现大约在口服给药后30至40分钟,肌肉注射后10至20分钟。所有给药途径的作用持续时间大约为3至4小时。在健康志愿者中口服给药后,平均口服
生物利用度约为12.5%。在这些患者中,达到最高血浆浓度的时间约为5小时。重复口服给药导致
氯丙嗪及其代谢物的积累。在多次每日两次给药后,
氯丙嗪的稳态在7天内达到。
来源:DrugBank
吸收、分配和排泄
氯丙嗪主要经粪便和胆汁排泄。尿液中可以检测到少量的未改变的
氯丙嗪及其代谢物。
来源:DrugBank
吸收、分配和排泄
在一项初步的药代动力学研究中,涉及健康志愿者,静脉注射6.25毫克和12.5毫克
氯丙嗪后,平均表观分布容积分别约为1401升和1548升。据报道,
氯丙嗪主要分布到大多数身体组织中,高浓度分布在肝脏和脾脏中。有证据表明,
吩噻嗪类药物会排入哺乳期母亲的乳汁中。
来源:DrugBank
吸收、分配和排泄
在健康志愿者中,注射给予
丙氯拉嗪的平均血浆清除率(CL)约为0.98升/小时 x 千克。平均肾清除率大约为23.6毫升/小时。
来源:DrugBank
吸收、分配和排泄
吩噻嗪类药物通常能很好地从胃肠道和注射部位吸收;然而,吸收可能不稳定,特别是在口服给药后。据报道,不同个体之间的峰值血浆浓度有显著差异。这种变异性可能是由于药物代谢速率的遗传差异、药物在胃肠道腔内的
生物降解和/或在吸收过程中(在胃肠道粘膜)和首次通过肝脏时的药物代谢所致。
来源:Hazardous Substances Data Bank (HSDB)