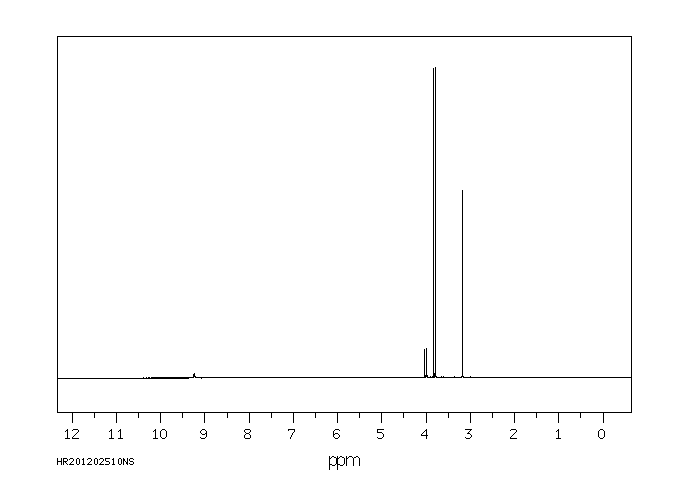
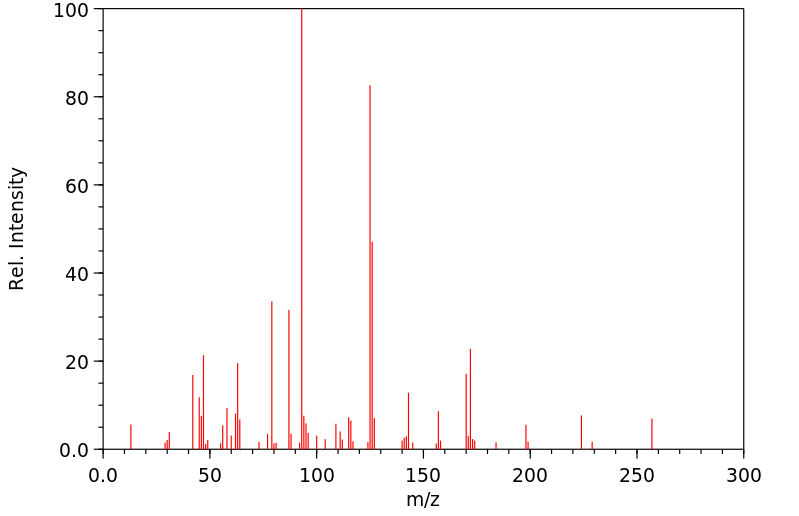
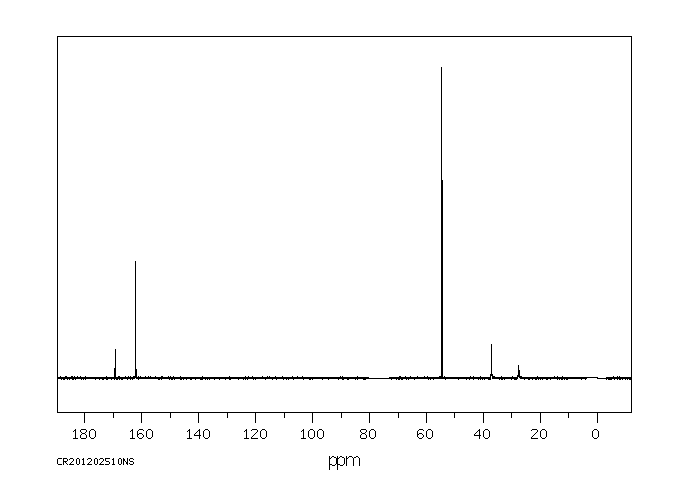
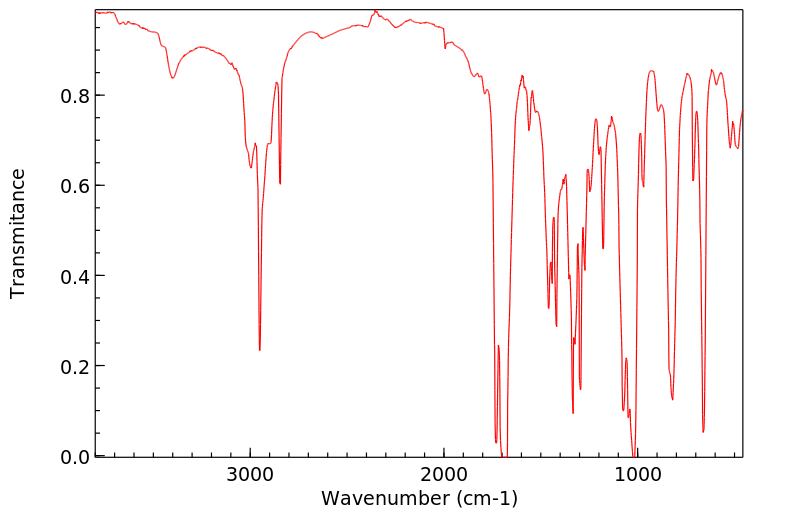
代谢
Metabolites include dimethoate, bis(dimethylthiophosphoryl)disulfide and dimethyldithiophosphorylacetic acid...
来源:Hazardous Substances Data Bank (HSDB)