对溴苯甲酰溴 | 18066-89-2
中文名称
对溴苯甲酰溴
中文别名
——
英文名称
1-bromo-4-(bromo(phenyl)methyl)benzene
英文别名
1-Bromo-4-[bromo(phenyl)methyl]benzene
CAS
18066-89-2
化学式
C13H10Br2
mdl
——
分子量
326.03
InChiKey
XQJCFOOAJAADSR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:361.8±27.0 °C(Predicted)
-
密度:1.638±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-溴苯甲酰苯 (4-bromophenyl)(phenyl)methanone 90-90-4 C13H9BrO 261.118 4-苄氧基苯甲酸 phenyl(4-bromophenyl)methanol 29334-16-5 C13H11BrO 263.134 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-溴二苯基甲烷 1-benzyl-4-bromo-benzene 2116-36-1 C13H11Br 247.134 4-溴苯甲酰苯 (4-bromophenyl)(phenyl)methanone 90-90-4 C13H9BrO 261.118
反应信息
-
作为反应物:参考文献:名称:苯海拉明的两种新的甲基和吗啉衍生物对大鼠的抗炎作用摘要:苯海拉明是乙醇胺类的第一代组胺H 1受体拮抗剂之一,具有许多药理特性,包括抗炎作用。在这项研究中,溴(II)和I的两个新的甲苯基衍生物(Di [对甲苯基] [二甲基氨基乙氧基]甲烷(III)和(Di [对甲苯基] [2-吗啉代乙氧基]甲烷(IV))合成。用福尔马林和组胺诱导的大鼠爪水肿评估了它们的急性和慢性抗炎活性。还测量了福尔马林和组胺引起的足爪水肿,二甲苯引起的耳部水肿以及在腹膜腔涂乙酸后的腹膜炎中的血管通透性,并与II进行了比较。选择棉丸诱导的肉芽肿模型来诱导大鼠慢性炎症。新合成的苯海拉明类似物似乎可以有效减少急性炎症。结论是,新药的显着抗炎作用可能与其降低血管通透性机制或对H 1组胺受体的拮抗作用有关。DOI:10.1007/s00044-011-9891-y
-
作为产物:参考文献:名称:N-Heterocyclic Carbene-Catalyzed Cross-Coupling of Aromatic Aldehydes with Activated Alkyl Halides摘要:N-Heterocyclic carbene-catalyzed umpolung of aldehydes followed by their interception with diarylbromomethanes has been reported. This conceptually novel transition-metal-free cross-coupling of aldehydes with alkyl halides works well at low catalyst loadings and under mild reaction conditions leading to the formation of diaryl acetophenone derivatives in good yields. In addition, a-halo ketones and esters can also be used, as aldehyde reaction partners.DOI:10.1021/ol102626p
文献信息
-
Kinetics of the Solvolyses of Benzhydryl Derivatives: Basis for the Construction of a Comprehensive Nucleofugality Scale作者:Bernard Denegri、André Streiter、Sandra Jurić、Armin R. Ofial、Olga Kronja、Herbert MayrDOI:10.1002/chem.200500845日期:2006.2.8series of 21 benzhydrylium ions (diarylmethylium ions) are proposed as reference electrofuges for the development of a general nucleofugality scale, where nucleofugality refers to a combination of leaving group and solvent. A total of 167 solvolysis rate constants of benzhydrylium tosylates, bromides, chlorides, trifluoroacetates, 3,5-dinitrobenzoates, and 4-nitrobenzoates, two-thirds of which have been提出了一系列21个苯甲鎓离子(二芳基甲基鎓离子)作为参考电熔剂,用于开发一般的核易性规模,其中核易性是指离去基团和溶剂的组合。甲苯磺酸苄酯,溴化物,氯化物,三氟乙酸盐,3,5-二硝基苯甲酸酯和4-硝基苯甲酸酯的共167个溶剂分解速率常数,在这项工作中已确定其中三分之二,根据相关方程log k(25摄氏度)= sf(Nf + Ef),其中sf和Nf是核反应堆特定的参数,Ef是电堆特定的参数。尽管以这种方式表征的核熔剂和电熔剂覆盖了12个数量级以上,但是只有一组参数,即sf,Nf和Ef,足以计算出25°C时的溶剂分解速率常数,精度为+/- 16%。到目前为止,由于所有核因子的sf大约为1,即离开基团/溶剂的组合,因此对核官能度的定性讨论可以基于Nf。
-
Nitrosoarene-Catalyzed HFIP-Assisted Transformation of Arylmethyl Halides to Aromatic Carbonyls under Aerobic Conditions作者:Suman Pradhan、Vishali Sharma、Indranil ChatterjeeDOI:10.1021/acs.orglett.1c02272日期:2021.8.6A rare metal-free nucleophilic nitrosoarene catalysis accompanied by highly hydrogen-bond-donor (HBD) solvent, 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP), organocatalytically converts arylmethyl halides to aromatic carbonyls. This protocol offers an effective means to access a diverse array of aromatic carbonyls with good chemoselectivity under mild reaction conditions. The activation of arylmethyl halides
-
Palladium-Catalyzed Synthesis of α-Carbonyl-α′-(hetero)aryl Sulfoxonium Ylides: Scope and Insight into the Mechanism作者:Christopher Janot、Jean-Baptiste Chagnoleau、Nathan R. Halcovitch、James Muir、Christophe AïssaDOI:10.1021/acs.joc.9b03032日期:2020.1.17these compounds over the parent diazo compounds. Herein, we report the palladium-catalyzed cross-coupling of aryl bromides and triflates with α-carbonyl sulfoxonium ylides. We also report the use of this method for the modification of an active pharmaceutical ingredient and for the synthesis of a key precursor of antagonists of the neurokinin-1 receptor. In addition, the mechanism of the reaction was尽管有最新进展,仍需要一种合成α-羰基-α'-(杂)芳基亚砜基磺酸盐的通用方法,以使这些化合物相对于母体重氮化合物具有更大的潜在安全优势。在本文中,我们报道了钯催化的芳基溴化物和三氟甲磺酸酯与α-羰基亚砜基鎓盐的交叉偶联。我们还报告了该方法用于修饰活性药物成分和合成神经激肽-1受体拮抗剂的关键前体的用途。此外,反应的机理是从几个观察结果中推断出来的。因此,通过31P 1H} NMR观察到氧化加成络合物[(XPhos)PhPdBr]及其二聚体,并且显示出这些络合物具有催化和动力学能力。而且,通过质谱法观察到由[(XPhos)ArPdBr](Ar = p-CF3-C6H4)与模型次硫酸on叶立德的金属转移所形成的络合物。最后,部分速率定律表明,在催化循环中,过渡金属化和随后的去质子化是速率决定的。
-
Synthesis and Anticonvulsant Activity of Some Cinnamylpiperazine Derivatives作者:Chuan Hu、Zhi-Gang Sun、Cheng-Xi Wei、Zhe-Shan QuanDOI:10.2174/157018010792929595日期:2010.11.1A series of cinnamylpiperazine derivatives was synthesized using different benzophenone as starting material. The structures of the compounds were proved by their IR, 1H-NMR spectroscopic data and mass spectra data. The anticonvulsant activities of these compounds were evaluated with maximal electroshock (MES) test and rotarod test with intraperitoneal injection on KunMing mice. Among all the flunarizine
-
Sulphide as a leaving group: highly stereoselective bromination of alkyl phenyl sulphides作者:Daniele Canestrari、Caterina Cioffi、Ilaria Biancofiore、Stefano Lancianesi、Lorenza Ghisu、Manuel Ruether、John O'Brien、Mauro F. A. Adamo、Hasim IbrahimDOI:10.1039/c9sc03560e日期:——A conceptionally novel nucleophilic substitution approach to synthetically important alkyl bromides is presented. Using molecular bromine (Br2), readily available secondary benzyl and tertiary alkyl phenyl sulphides are converted into the corresponding bromides under exceptionally mild, acid- and base-free reaction conditions. This simple transformation allows the isolation of elimination sensitive提出了一种概念上新颖的对重要合成烷基溴的亲核取代方法。使用分子溴(Br2),在异常温和,无酸和无碱的反应条件下,容易获得的仲苄基和叔烷基苯基硫化物转化为相应的溴化物。这种简单的转化可以以高收率和高纯度分离出对消除敏感的苄基β-溴羰基和腈化合物。值得注意的是,质子官能团(例如酸和醇)是可以容忍的。通过不对称磺胺-迈克尔加成反应很容易制得的对映体富集的苄基β-磺基酯,可产生相应的具有高立体选择性的倒置溴化物,在-40°C时达到完全对映体特异性。显着地,报道的苄基β-溴代酯可以在没有消旋作用的情况下在-20°C下长时间保存。一锅法制备90%ee的γ-叠氮基醇(S)-5证明了其合成潜力。NMR研究表明,最初形成了硫化物溴加合物,而该加合物又与假定的经历C-Br键形成的二溴硫丙烷中间体处于平衡状态。
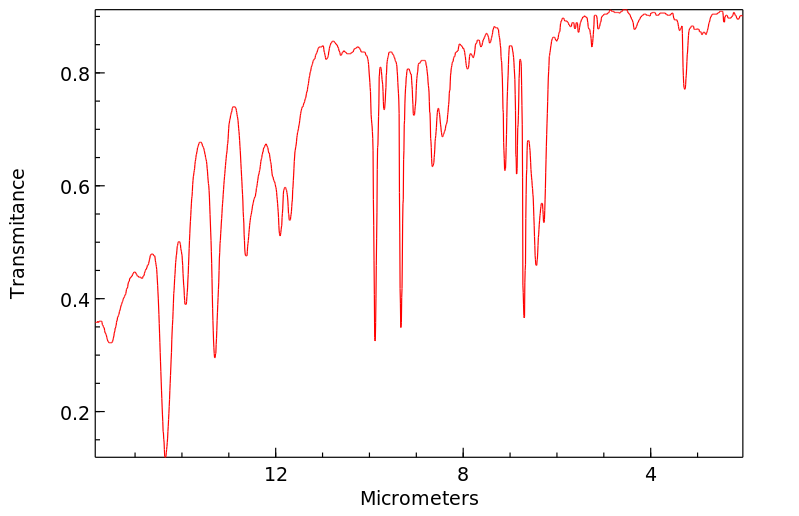
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫