己二酸二(2-乙基己)酯 | 103-23-1
中文名称
己二酸二(2-乙基己)酯
中文别名
己二酸二辛脂;己二酸二异辛酯;己二酸二(2-乙基己基)酯;已二酸二辛酯;己二酸二乙基己基酯;己二酸二辛酯;己二酸(2-乙基己酯);DOA
英文名称
di(2-ethylhexyl)adipate
英文别名
hexanedioic acid bis(2-ethylhexyl) ester;bis(2-ethylhexyl)adipate;bis(2-ethylhexyl) hexanedioate;DEHA;Bis(2-ethylhexyl) adipate
CAS
103-23-1
化学式
C22H42O4
mdl
MFCD00009496
分子量
370.573
InChiKey
SAOKZLXYCUGLFA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-67 °C
-
沸点:417 °C
-
密度:0.925 g/mL at 20 °C(lit.)
-
闪点:>230 °F
-
溶解度:<0.0001克/升
-
LogP:8.94 at 25℃
-
物理描述:Bis(2-ethylhexyl) adipate is a colorless to straw-colored liquid with a mild odor. Floats on water. (USCG, 1999)
-
颜色/状态:COLORLESS OR VERY PALE AMBER LIQ
-
气味:SLIGHT AROMATIC SMELL
-
蒸汽密度:12.8 (NTP, 1992) (Relative to Air)
-
蒸汽压力:8.5X10-7 mm Hg at 20 °C
-
亨利常数:4.34e-07 atm-m3/mole
-
自燃温度:710 °F (377 °C)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:13.7 cP @ 20 °C
-
燃烧热:-15,430 Btu/lb= -8,580 cal/g= -359X10+5 Joules/kg
-
汽化热:1.2542X10+8 J/kmol at -111.66 °C
-
折光率:Index of refraction: 1.4474 @ 20 °C/D
-
保留指数:2382;2390;2382;2381;2383;2361;2381;2381;2383
-
稳定性/保质期:
避免与强氧化剂接触。这是一种较为稳定的化合物。
计算性质
-
辛醇/水分配系数(LogP):6.8
-
重原子数:26
-
可旋转键数:19
-
环数:0.0
-
sp3杂化的碳原子比例:0.909
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
ADMET
代谢
在大鼠体内和体外对增塑剂DEHA的代谢进行了研究,以确定肝脏过氧化物酶体增殖剂浓度变化所涉及的不同步骤。在体内研究中,通过灌胃给Wistar大鼠不同剂量的DEHA和单-(2-乙基己基)-己二酸酯,持续5天。在体外研究中,通过原位灌注分离出肝细胞。在大鼠给药后24小时内,尿液中没有检测到DEHA;己二酸是主要的代谢物。只有2-乙基己醇途径显示出进一步的代谢物,主要是2-乙基己酸,它要么被结合,要么进入其他途径。虽然2-乙基己酸葡萄糖醛酸化似乎与剂量和时间有关,但2-乙基己醇葡萄糖醛酸化更为稳定。在体外,DEHA的首次水解似乎是一个速率限制步骤。当单-(2-乙基己基)己二酸酯直接添加到培养基中时,发现了体内研究中识别的所有代谢物。2-乙基己醇和2-乙基己酸的葡萄糖醛酸化与剂量和时间有关。
In vivo and in vitro metabolism of the plasticizer DEHA was examined in the rat to determine the different steps involved in the hepatic concn of peroxisomal proliferators. In the in vivo studies, different doses of DEHA and mono-(2-ethylhexyl)-adipate were administered by gavage to Wistar rats for 5 days. In the in vitro studies, hepatocytes were isolated by in situ perfusion. No DEHA was recovered in rat urine 24 hr after administration; adipic acid was the main metabolite. Only the 2-ethylhexanol pathway showed further metabolites, mainly 2-ethylhexanoic acid which was either conjugated or submitted to other pathways. While 2-ethylhexanoic acid glucuronidation appeared to be dose and time dependent, 2-ethylhexanol glucuronidation was more stable. In vitro, the first hydrolysis of DEHA appeared to be a rate limiting step. When mono-(2-ethylhexyl) adipate was added directly to the culture medium, all the metabolites identified in the in vivo study were found. Glucuronidation of both 2-ethylhexanol and 2-ethylhexanoic acid was dose and time dependent.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
没有关于人类的数据。动物致癌性证据有限。总体评估:第3组:该物质对人类致癌性无法分类。
No data are available in humans. Limited evidence of carcinogenicity in animals. OVERALL EVALUATION: Group 3: The agent is not classifiable as to its carcinogenicity to humans.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
分类:C;可能的人类致癌物。分类依据:基于缺乏人类数据以及雌性小鼠肝脏肿瘤发生率增加。除了阳性显性致死分析外,没有遗传毒性的证据;然而,该化合物与其他被归类为可能和可能的非遗传毒性人类致癌物的化合物存在结构关系。人类致癌性数据:无。动物致癌性数据:有限。
CLASSIFICATION: C; possible human carcinogen. BASIS FOR CLASSIFICATION: Based on an absence of human data and increased incidence of liver tumors in female mice. Except for a positive dominant lethal assay, there was no evidence of genotoxicity; this compound does, however, exhibit structural relationships to other nongenotoxic compounds classified as probable and possible human carcinogens. HUMAN CARCINOGENICITY DATA: None. ANIMAL CARCINOGENICITY DATA: Limited.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:己二酸二(2-乙基己基)酯
IARC Carcinogenic Agent:Di(2-ethylhexyl) adipate
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:对其对人类的致癌性无法分类
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第7卷补充:致癌性的总体评估:更新国际癌症研究机构专著第1至42卷,1987年;440页;ISBN 92-832-1411-0(已绝版)
IARC Monographs:Volume Sup 7: Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42, 1987; 440 pages; ISBN 92-832-1411-0 (out of print)
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
研究了DEHA在大鼠和小鼠中的吸收、分布和消除。雄性Sprague Dawley大鼠、雄性NMRI小鼠以及在妊娠第17天的雌性NMRI小鼠被给予溶解在二甲基亚砜或玉米油中的(14)C标记的DEHA,通过静脉注射或口服给药。DEHA在羰基或醇基团上进行了标记。动物在给药后5分钟至4天内被处死,通过全身自动放射性显影确定(14)C活性的组织分布。来自羰基标记DEHA的(14)C活性在所有动物中的分布相似。在口服或静脉给药后,体内脂肪、肝脏和肾脏的放射性水平最高。来自醇标记DEHA的(14)C活性在雄性小鼠的支气管中发现。在怀孕小鼠中,在给予羰基标记DEHA后的前24小时内,(14)C活性在胎儿肝脏、肠道和骨髓中被观察到。给予醇标记DEHA的小鼠的胎儿中几乎未发现放射性标记。在给药后4天,小鼠中没有发现来自DEHA的放射性活性。大鼠血液中DEHA的浓度在给予二甲基亚砜而不是玉米油时增加更快,浓度是后者的两到三倍。在用二甲基亚砜处理的DEHA的大鼠中,大量的DEHA通过胆汁排出。在给予羰基标记DEHA的动物中,胆汁排泄的放射性标记非常少。DEHA通过尿液排出,但在进行胆汁收集实验的动物中,排出量较小。溶剂对排出量影响很小。DEHA从油溶液中的吸收较差。
The absorption, distribution, and elimination of DEHA were studied in mice and rats. Male Sprague Dawley rats, male NMRI mice, and pregnant female NMRI mice on day 17 of gestation were administered (14)C labeled DEHA dissolved in dimethyl sulfoxide or corn oil iv or intragastrically. The DEHA was labeled on the carbonyl or alcohol moiety. Animals were killed 5 min to 4 days after dosing, and the tissue distribution of (14)C activity was determined by whole body autoradiography. The tissue distribution of (14)C activity from carbonyl labeled DEHA was similar in all animals. Highest levels of radioactivity were observed in the body fat, liver, and kidney after intragastrically or iv administration. (14)C activity from alcohol labeled DEHA was found in the bronchi of male mice. In pregnant mice, (14)C activity was observed in the fetal liver, intestine, and bone marrow during the first 24 hr after carbonyl labeled DEHA was given. Very little radiolabel was found in fetuses of mice given alcohol labeled DEHA. No DEHA derived radioactivity was found in mice 4 days after dosing. Blood DEHA concn in rats increased faster and were two or three times higher when the dose was given in DMSO rather than corn oil. Significant amounts of DEHA were excreted in the bile of rats treated with DEHA in DMSO. Very little biliary elimination of radiolabel occurred in animals given carbonyl labeled DEHA. DEHA was excreted in the urine, the amounts being smaller in animals used in the bile collection experiments. The vehicle had very little effect on the amount excreted. DEHA is poorly absorbed from an oil solution.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:9
-
危险品标志:Xn
-
安全说明:S26,S36,S36/37,S45
-
危险类别码:R40
-
WGK Germany:1
-
海关编码:29171290
-
危险品运输编号:UN 1230 3/PG 2
-
RTECS号:AU9700000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:储存于阴凉、通风的库房。远离火种、热源,防止阳光直射。保持容器密封,并与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 己二酸二-2-乙基己酯;己二酸二辛酯 |
| 化学品英文名称: | Di(2-ethylhcxyl)adipate;Dioctyl adipate |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 103-23-1 |
| 分子式: | C 22 H 42 O 4 |
| 分子量: | 370.64 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:己二酸二-2-乙基己酯;己二酸二辛酯 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 本品对眼睛、皮肤有轻微刺激作用。至今,未见中毒病例报告。 |
| 环境危害: | 对环境有危害,对水体可造成污染。 |
| 燃爆危险: | 本品可燃,具弱刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 用肥皂水及清水彻底冲洗。就医。 |
| 眼睛接触: | 拉开眼睑,用流动清水冲洗15分钟。就医。 |
| 吸入: | 脱离现场至空气新鲜处。就医。 |
| 食入: | 误服者,饮适量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。受高热分解放出有毒的气体。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 尽可能将容器从火场移至空旷处。喷水保持火场容器冷却,直至灭火结束。处在火场中的容器若已变色或从安全泄压装置中产生声音,必须马上撤离。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | |
| 禁止使用的灭火剂: | 不宜用水。 |
| 闪点(℃): | 181 |
| 自燃温度(℃): | 235 |
| 爆炸下限[%(V/V)]: | |
| 爆炸上限[%(V/V)]: | |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,建议应急处理人员戴好口罩、护目镜,穿工作服。用砂土吸收,收集运至废物处理场所。也可以用大量水冲洗,经稀释的洗水放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,全面通风。防止蒸气泄漏到工作场所空气中。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿透气型防毒服,戴乳胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。在清除液体和蒸气前不能进行焊接、切割等作业。避免产生烟雾。避免与氧化剂接触。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。防止阳光直射。保持容器密封。应与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中 国 MAC:未制订标准前苏联 MAC:未制订标准美国TLV—TWA:未制订标准 |
| 监测方法: | |
| 工程控制: | 提供良好的自然通风条件。 |
| 呼吸系统防护: | 作业工人应戴口罩。 |
| 眼睛防护: | 一般不需要特殊防护,高浓度接触时可戴安全防护眼镜。 |
| 身体防护: | 穿工作服。 |
| 手防护: | 一般不需特殊防护。 |
| 其他防护: | 工作后,淋浴更衣。 |
| 第九部分:理化特性 |
| 外观与性状: | 无色或微黄色油状液体,有特殊气味。 |
| pH: | |
| 熔点(℃): | -67.8 |
| 沸点(℃): | 417 |
| 相对密度(水=1): | 0.927(20℃) |
| 相对蒸气密度(空气=1): | |
| 饱和蒸气压(kPa): | 0.32/200℃ |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 181 |
| 引燃温度(℃): | 235 |
| 爆炸上限%(V/V): | |
| 爆炸下限%(V/V): | |
| 分子式: | C 22 H 42 O 4 |
| 分子量: | 370.64 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 微溶于水,溶于多数有机溶剂。 |
| 主要用途: | 用作增塑剂或特种润滑剂及气相色谱固定液。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:9100mg/kg(大鼠经口) LC50:无资料 |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | 家兔经眼:开放性刺激试验, 500mg,引起刺激。家兔经眼:500mg/24小时,轻度刺激。 |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 建议用焚烧法处置。在能利用的地方重复使用容器或在规定场所掩埋。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | |
| UN编号: | |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | |
| 运输注意事项: | 储存于阴凉、通风仓间内。远离火种、热源。保持容器密封。防止阳光曝晒。应与氧化剂分开存放。禁止使用易产生火花的机械设备和工具。搬运时要轻装轻卸,防止包装及容器损坏。分装和搬运作业要注意个人防护。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 5 |
| MSDS修改日期: | 年月日 |
制备方法与用途
化学性质
无色或微黄色油状液体。沸点在0.67kPa时为210℃,在0.27kPa时为175℃,在0.130kPa时为167℃。相对密度为0.990(20/4℃),折光率为1.4470。闪点为193℃。溶于多数有机溶剂,微溶于乙二醇类,不溶于水。有特殊气味。
用途
该品是聚氯乙烯的优良耐寒增塑剂,赋予制品良好的低温柔软性,并具有一定的光热稳定性和耐水性。在塑溶胶中,初期粘度较低且稳定性良好。常与DOP等主增塑剂并用于耐寒农用薄膜、电线、薄板、人造革、户外用水管以及冷冻食品的包装薄膜。还可作为许多合成橡胶的低温增塑剂以及硝基纤维素、乙基纤维素等树脂的增塑剂。在实验室工作中可用作气相色谱固定液。己二酸二(2-乙基己基)酯的毒性较低,对人的致死量约为500,000 mg/kg,对大鼠口服LD₅₀为3000-6000 mg/kg,对皮肤及眼睛的刺激也很轻微。
用途
用作多种树脂特别是聚氯乙烯和氯乙烯共聚物的耐寒增塑剂,也可作为航空润滑脂的原料。此外,还可用于气相色谱固定液(最高使用温度135℃,溶剂为乙醚)、增塑剂、溶剂等用途。
生产方法
由己二酸和2-乙基己醇在硫酸催化下减压酯化而得。工业品己二酸二(2-乙基己基)酯含量≥99%。原料消耗量:己二酸 415 kg/t,2-乙基己醇 750 kg/t。
类别
有毒物品
毒性分级
低毒
急性毒性
口服-大鼠 LD₅₀: 9110 毫克/公斤;口服-小鼠 LD₅₀:15000 毫克/公斤
刺激数据
皮肤-兔子 500 毫克 轻度;眼睛-兔子 500 毫克 中度
可燃性危险特性
遇明火、高温或强氧化剂可燃;燃烧排放刺激烟雾
储运特性
库房通风低温干燥,与食品原料分开存放
灭火剂
泡沫、干粉、二氧化碳、砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙基己基己二酸氢酯 Hexanedioic acid, mono(2-ethylhexyl) ester 4337-65-9 C14H26O4 258.358
反应信息
-
作为反应物:描述:己二酸二(2-乙基己)酯 、 苯胺 在 甲烷磺酸 、 氢气 、 tris(acetylacetonato)ruthenium(III) 、 1,1,1-三(二苯基膦甲基)乙烷 作用下, 以 1,4-二氧六环 为溶剂, 220.0 ℃ 、1.0 MPa 条件下, 反应 42.0h, 以80%的产率得到1-苯基氮杂环庚烷参考文献:名称:苯胺存在下二酯加氢制备 N-芳香杂环的新路线摘要:二羧酸及其酯在苯胺存在下的氢化提供了杂环的新合成。[Ru(acac)3] 和 1,1,1-三(二苯基膦甲基)乙烷 (triphos) 在 220 °C 下产生良好至优异的环胺产率。当氨水与二甲基1,6-己二烯酸一起使用时,以良好的收率获得了γ-己内酰胺。通过使用衍生自较长链和支链醇的二酯,可以抑制涉及甲醇对胺进行烷基化的副反应。光学纯二酯((R)-2-甲基丁二酸二甲酯和(S)-2-甲基丁二酸二甲酯)与苯胺氢化得到外消旋3-甲基-1-苯基吡咯烷,产率78%。DOI:10.1039/c7sc01718a
-
作为产物:参考文献:名称:具有非共价固定在多壁碳纳米管上的脂肪酶的异常活性和可重复使用的纳米生物催化剂,用于合成二酯增塑剂摘要:已经开发了一种新的合成二羧酸酯的方法,该方法是在一种新型多相纳米生物催化剂的存在下,该催化剂由固定在多壁碳纳米管(MWCNT)上的南极假丝酵母脂肪酶B组成。最初进行了南极假丝酵母脂肪酶B(CALB)支持物的选择和表征。测试了具有不同几何形状,形态和表面/核心修饰的原始和修饰的MWCNTs作为脂肪酶载体,并确定了CALB负载量为15.7 wt%的CheapTubes ™ MWCNTs纳米生物催化剂是活性最高的纳米生物催化剂。琥珀酸与n的模型反应在环己烷中,在45°C下,每1 mmol琥珀酸中加入4摩尔过量的醇和150 mg的纳米生物催化剂,进行丁醇反应。3小时后以95%的产率获得琥珀酸二正丁酯。将新的纳米生物催化剂的活性与基准的Novozyme-435和其他酸性催化剂进行了比较。回收研究表明,可以使用六次活性最高的MWCNTs-脂肪酶生物催化剂,而不会造成任何明显的活性损失。这项研究的主要优DOI:10.1016/j.apcata.2019.01.030
-
作为试剂:描述:2-chloro-4-(3-(4-fluorophenyl)-5-(methylthio)-4H-1,2,4-triazole-4-yl)pyridine 、 间硝基苯胺 在 己二酸二(2-乙基己)酯 、 caesium carbonate 、 4,5-双二苯基膦-9,9-二甲基氧杂蒽 、 bis(dibenzylideneacetone)-palladium(0) 作用下, 以 1,4-二氧六环 为溶剂, 以76.1%的产率得到4-(3-(4-fluorophenyl)-5-(methylthio)-4H-1,2,4-triazol-4-yl)-N-(3-nitrophenyl)pyridin-2-amine参考文献:名称:2-氨基-4-取代吡啶衍生物及其合成方法和应 用摘要:本发明公开了一种2‑氨基‑4‑取代吡啶衍生物及其合成方法和应用。该衍生物的结构通式为具有该结构的化合物,或其药学可接受的盐、水合物、溶剂合物、多晶型物、互变异构体、立体异构、同位素衍生物或前药具有较高的EGFR抑制活性,对人表皮细胞癌细胞以及胶质母细胞瘤细胞等具有较强的抑制活性,可用于制备治疗和/或预防和/或延缓和/或辅助治疗和/或处理与EGFR活性过高相关的疾病的药物。公开号:CN110041302B
文献信息
-
[EN] CONVERSION OF 5-(HALOMETHYL)-2-FURALDEHYDE INTO POLYESTERS OF 5-METHYL-2FUROIC ACID<br/>[FR] CONVERSION DE 5- (HALOMÉTHYL) -2-FURALDÉHYDE EN POLYESTERS D'ACIDE 5-MÉTHYL-2-FUROÏQUE申请人:XF TECHNOLOGIES INC公开号:WO2015179088A1公开(公告)日:2015-11-26The present invention provides methods of synthesis of di-, tri-, and poly- ester derivatives of 5- methyl-2-furoic acid; di-, tri-, and poly- amide derivatives of 5-methyl-2-furoic acid; and di-, tri-, and poly- thioester derivatives of 5-methyl-2-furoic acid from 5-(halomethyl)-2-furaldehydes. These molecules are useful as plasticizers, solvents, coalescers, an ingredient in plastisol compositions, diluents, an ingredient in adhesive compositions, compatibilizer or compounding agent, or lubricant, as well as polymer compositions containing such ester compositions.
-
[EN] PHOTOCHROMIC AND ELECTROCHROMIC DIARYLETHENE COMPOUNDS WITH IMPROVED PHOTOSTABILITY AND SOLUBILITY<br/>[FR] COMPOSÉS DIARYLÉTHÈNE PHOTOCHROMIQUES ET ÉLECTROCHROMES PRÉSENTANT UNE PHOTOSTABILITÉ ET UNE SOLUBILITÉ AMÉLIORÉES申请人:SWITCH MAT INC公开号:WO2020198868A1公开(公告)日:2020-10-08A diarylethene compound reversibly convertible under photochromic and electrochromic conditions between a ring-open isomer of Formula (1A) and a ring-closed isomer of Formula (IB) wherein R5 is a substituted phenyl ring and Re is a substituted thiophene ring is provided. The photochromic-electrochromic diarylethene compound of Formula (1A)/(1B) have improved photochromic, electrochromic or photochromic and electrochromic properties, and is useful to provide variation of the light transmission properties of optical filters. The compound also possesses improved solubility making it suitable for incorporation in commercial products..
-
Process for the preparation of olmesartan medoxomil申请人:KRKA, tovarna zdravil, d.d., Novo mesto公开号:EP1816131A1公开(公告)日:2007-08-08The present invenion relates to an improved process for the manufacture of olmesartan and pharmaceutically acceptable salts and esters thereof as an active ingredient of a medicament for the treatment of hypertension and related diseases and conditions.本发明涉及一种改进的工艺,用于制造奥美沙坦及其药用可接受的盐和酯,作为治疗高血压及相关疾病和症状的药物的活性成分。
-
[EN] HEMI-AMINAL ETHERS AND THIOETHERS OF N-ALKENYL CYCLIC COMPOUNDS<br/>[FR] ÉTHERS ET THIOÉTHERS HÉMIAMINAUX DE COMPOSÉS CYCLIQUES N-ALCÉNYLIQUES申请人:ISP INVESTMENTS INC公开号:WO2014116560A1公开(公告)日:2014-07-31Described herein are hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds that may be produced through a reaction comprising: (A) at least one first reactant represented by a structure (I), wherein X is a functionalized or unfunctionalized C1-C5 alkylene group optionally having one or more heteroatoms, and each R1, R2, and R3 is independently selected from the group consisting of hydrogen and functionalized and unfunctionalized alkyl groups optionally having one or more heteroatoms, and (B) at least one second reactant having at least one hydroxyl moiety or thiol moiety. The hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds may comprise a polymerizable moiety, in which case they may be left as-is or used to create homopolymers or non-homopolymers, or they may not comprise a polymerizable moiety. A wide variety of formulations may be created using the hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds, including personal care, oilfield, and construction formulations.
-
Hexahydrophthalate based compound and process for producing the same申请人:Shieh Sung-Yueh公开号:US20090281349A1公开(公告)日:2009-11-12A hexahydrophthalate based compound is adapted to use as a plasticizer that contains no phthalic acid and benzoic acid, possess physical properties superior to DEHA and DINA in transparency and adhesion and is friendly to organisms and the environment; and a process for producing the hexahydrophthalate based compound includes esterifying hexahydrophthalic anhydride, a diol, and a catalyst for decarboxylation to get hexahydrophthalic alcohol, and adding a monoacid into the hexahydrophthalic alcohol for further esterification, thereby obtaining the hexahydrophthalate based compound.
表征谱图
-
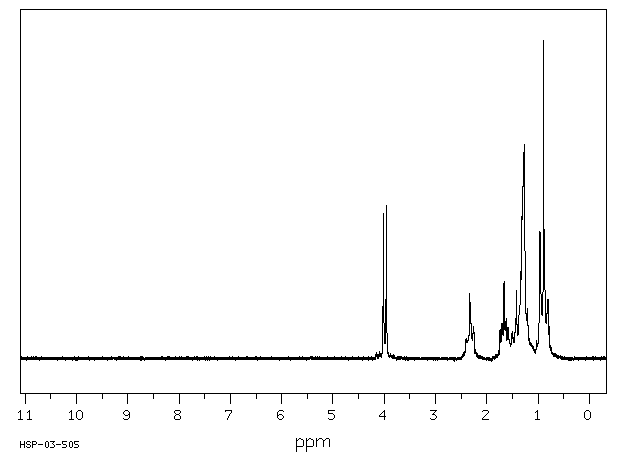
氢谱1HNMR
-
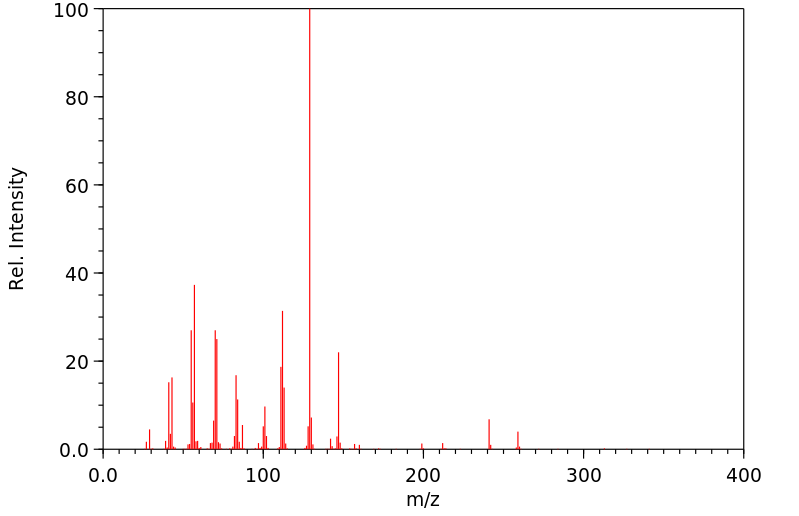
质谱MS
-
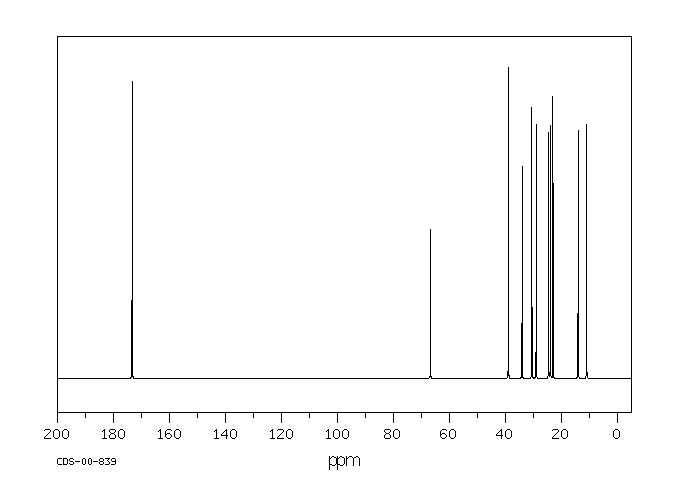
碳谱13CNMR
-
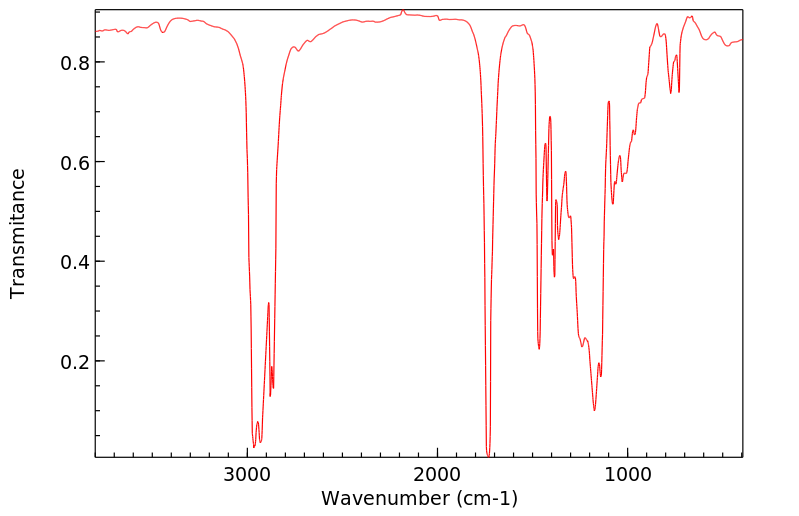
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯