(3E)-4-(4-羟基苯基)-3-丁烯-2-酮 | 22214-30-8
中文名称
(3E)-4-(4-羟基苯基)-3-丁烯-2-酮
中文别名
——
英文名称
(E)-4-(4-hydroxyphenyl)-3-buten-2-one
英文别名
(E)-4-(4-hydroxyphenyl)but-3-en-2-one;4-hydroxybenzylideneacetone;4(4-hydroxy-phenyl)-but-3-en-2-one;(E)-4-(4-hydroxyphenyl)-3-butylene-2-one;(3E)-4-(4-hydroxyphenyl)but-3-en-2-one;(1E)-1-(4-hydroxyphenyl)but-1-en-3-one;p-hydroxybenzylideneacetone;p-Hydroxybenzalacetone
CAS
22214-30-8
化学式
C10H10O2
mdl
MFCD00016490
分子量
162.188
InChiKey
OCNIKEFATSKIBE-NSCUHMNNSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
储存条件:存储条件:密封、干燥、避光,适宜温度为2-8°C。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 反-4-(4-甲氧苯基)-3-丁烯-2-酮 4-(4-methoxyphenyl)-3-buten-2-one 3815-30-3 C11H12O2 176.215 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (1E,4E)-1-(4-hydroxyphenyl)-5-phenylpenta-1,4-dien-3-one —— C17H14O2 250.297 —— (1E,4E)-1-(4-hydroxyphenyl)-5-(4-methoxyphenyl)penta-1,4-dien-3-one —— C18H16O3 280.323 —— 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one 149732-52-5 C19H16O3 292.334 4-乙酰氧基亚苄基丙酮 4-(p-acetoxyphenyl)but-3-en-2-one 41437-95-0 C12H12O3 204.225 —— 1-(4-hydroxyphenyl)-7-(3-hydroxyphenyl)-1,4,6-heptatrien-3-one 1612860-82-8 C19H16O3 292.334 —— (1E,4E)-1-(4-hydroxyphenyl)-5-(3-methoxyphenyl)penta-1,4-dien-3-one —— C18H16O3 280.323 —— 4-[5-(4-hydroxyphenyl)-3-oxopenta-1,4-dienyl]benzoic acid —— C18H14O4 294.307 —— 1-(4-hydroxyphenyl)-7-(2-hydroxyphenyl)-1,4,6-heptatrien-3-one 1612860-83-9 C19H16O3 292.334
反应信息
-
作为反应物:描述:(3E)-4-(4-羟基苯基)-3-丁烯-2-酮 在 喹啉 、 氢气 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 反应 19.0h, 生成 5-[(E)-2-(4-hydroxyphenyl)ethenyl]-5-methyl-2,5-dihydrofuran-2-one参考文献:名称:Design, synthesis and in vitro evaluation against human cancer cells of 5-methyl-5-styryl-2,5-dihydrofuran-2-ones, a new series of goniothalamin analogues摘要:The present work describes the preparation of a novel series of compounds based on the structure of goniothalamin (1), a natural styryl lactone with known cytotoxic and antiproliferative activities against a variety of cancer cell lines. A focused library of 17 goniothalamin analogues displaying the 5-methyl-2,5-dihydrofuran-2-one motif were prepared, and their cytotoxicity evaluated. While the analogues bearing methoxy and/or hydroxy groups on the aromatic moiety usually were at least three times less potent than the lead compound (1), ortho and para-trifluoromethyl analogues 10 and 11 exhibited levels of cytotoxicity similar to goniothalamin (1) against most cancer cell lines evaluated. One could suggest that the electronic effect of the trifluoromethyl group activates the inhibitor's electrophilic site via reduction of the electron density of the alpha,beta-unsaturated ester oxygen atom. These results provide new information on the structure activity relationship of these alpha,beta-unsaturated styryl lactones, thereby further focusing the design of novel candidates. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2013.06.044
-
作为产物:描述:1-(4-羟基苯基)-3-丁烯醇 在 chromium(VI) oxide 、 盐酸 、 palladium dichloride 作用下, 以 水 、 乙腈 为溶剂, 反应 1.0h, 生成 (3E)-4-(4-羟基苯基)-3-丁烯-2-酮参考文献:名称:痕量OH导向的Wacker氧化消除反应,替代Wittig烯烃/醇醛缩合反应:由均烯丙基醇一锅法合成α,β-不饱和和非共轭酮摘要:用于一锅合成的新方法β-取代和β,β -二取代的α,β通过顺序的PdCl不饱和的甲基酮从高烯丙基醇2 / CRO 3促进的瓦克法然后酸介导的脱水反应已经开发。值得注意的是,内部均烯丙基醇在相同的方案下可选择性地引入区域选择性的非共轭不饱和羰基化合物。证明了一种新的基于原料的α,β-不饱和和非共轭甲基酮的合成方法。DOI:10.1021/acs.joc.6b01899
文献信息
-
Direct Organocatalytic Asymmetric Heterodomino Reactions: The Knoevenagel/Diels−Alder/Epimerization Sequence for the Highly Diastereoselective Synthesis of Symmetrical and Nonsymmetrical Synthons of Benzoannelated Centropolyquinanes作者:D. B. Ramachary、K. Anebouselvy、Naidu S. Chowdari、Carlos F. BarbasDOI:10.1021/jo049581r日期:2004.9.1Amino acids and amines have been used to catalyze three component hetero-domino Knoevenagel/Diels−Alder/epimerization reactions of readily available various precursor enones (1a−l), aldehydes (2a−p), and 1,3-indandione (3). The reaction provided excellent yields of highly substituted, symmetrical and nonsymmetrical spiro[cyclohexane-1,2‘-indan]-1‘,3‘,4-triones (5) in a highly diastereoselective fashion氨基酸和胺已用于催化易得的各种前体烯酮(1a - l),醛(2a - p)和1,3-茚满二酮(3)的三组分异多米诺Knoevenagel / Diels-Alder /异构化反应。该反应以对映体选择性低至中等的高度非对映选择性提供了极高产率的高度取代的,对称和非对称的螺环己烷-1,2 -'-茚满-1',3',4-三酮(5)。芳基醛(2a - p)和1,3-茚满二酮(3)的Knoevenagel缩合反应在有机催化下提供了亚芳基1,3-茚满二酮(17),收益率很高。我们首次证明了反式-螺烷(6)对顺式螺烷(5)的氨基酸和胺催化的差向异构反应。反式-螺环烷(6)转化为顺式螺环烷5的机理显示为通过逆迈克尔/迈克尔反应进行,而不是通过分离顺式螺环烷的吗啉烯胺中间体(22)进行去质子化/再质子化。前手性顺式螺环烷(5ab)和反式螺环烷(6ab)是合成苯甲酰化的中心聚奎宁烷的极好原料。在氨基酸和胺的催化
-
Synthesis of Enones and Enals via Dehydrogenation of Saturated Ketones and Aldehydes作者:Gao-Fei Pan、Xue-Qing Zhu、Rui-Li Guo、Ya-Ru Gao、Yong-Qiang WangDOI:10.1002/adsc.201801058日期:2018.12.21substrate scope including various linear or cyclic saturated ketones and aldehydes. The protocol is ligand‐free, and molecular oxygen is used as the sole clean oxidant in the reaction. Due to mild reaction conditions, good functional group compatibility, and versatile utilities of enones and enals, the method can be applied in the late‐stage synthesis of natural products, pharmaceuticals and fine chemicals
-
Cobalt-Catalyzed Carbonylative Cross-Coupling of Alkyl Tosylates and Dienes: Stereospecific Synthesis of Dienones at Low Pressure作者:Brendon T. Sargent、Erik J. AlexanianDOI:10.1021/jacs.7b07983日期:2017.9.13carbonylative coupling of alkyl tosylates and dienes producing enantioenriched dienones. This catalytic process proceeds under low pressure and mild conditions using a simple cobalt catalyst and extends to diverse tosylate and diene coupling partners. The transformation constitutes a unique, convergent approach to the asymmetric synthesis of valuable carbonyl compounds from easily accessed starting materials
-
Synthesis and antiviral bioactivity of novel (1E, 4E)-1-aryl-5-(2-(quinazolin-4-yloxy)phenyl)-1,4-pentadien-3-one derivatives作者:Hui Luo、Jiaju Liu、Linhong Jin、Deyu Hu、Zhen Chen、Song Yang、Jian Wu、Baoan SongDOI:10.1016/j.ejmech.2013.02.035日期:2013.5A series of novel (1E, 4E)-1-aryl-5-[2-(quinazolin-4-yloxy)phenyl]-1,4-pentadien-3-one derivatives were designed and synthesized by reacting substituent aldehyde with intermediates 4a–f. Antiviral bioassays indicated that most of the compounds exhibited promising ex vivo antiviral bioactivities against tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV) at 500 μg/mL. The relationship between通过取代醛与苯甲醛的反应合成了一系列新颖的(1 E,4 E)-1-芳基-5- [2-(喹唑啉-4-基氧基)苯基] -1,4-戊二烯-3-酮衍生物。中间体4a – f。抗病毒生物测定表明,大多数化合物在500μg/ mL的浓度下对烟草花叶病毒(TMV)和黄瓜花叶病毒(CMV)表现出有希望的离体抗病毒生物活性。还讨论了结构与抗病毒活性之间的关系。化合物5a,6e和6g在离体TMV上可具有大约50%的明显保护性生物活性(EC 50)分别为257.7、320.7和243.3μg/ mL。该研究首次证明(1 E,4 E)-1-芳基-5-(2-(喹唑啉-4-基氧基)苯基)-1,4-戊二烯-3-酮可用于开发潜力植物用杀病毒剂。
-
Antiviral 1-aryl-5-amino-1-penten-3-ones申请人:Merrell Dow Pharmaceuticals Inc.公开号:US04400380A1公开(公告)日:1983-08-23Compounds of the formula ##STR1## wherein R is H, halo, C.sub.1-4 alkyl, C.sub.1-4 alkoxy, 3,4-methylenedioxy, CF.sub.3, NO.sub.2, NH.sub.2, N(C.sub.1-4 alkyl).sub.2, CN, OH, --S(C.sub.1-4 alkyl) or --SO.sub.2 (C.sub.1-4 alkyl); R.sub.1 and R.sub.2 are independently each H or C.sub.1-4 alkyl or taken together with the attached N atom are morpholino, piperidino, pyrrolidino, piperazino, or N-- C.sub.1-4 -alkyl piperazino; and, when R is halo, C.sub.1-4 alkyl or C.sub.1-4 alkoxy, x is 0-3 and, otherwise, is 0 or 1; or a pharmaceutically acceptable salt thereof with an acid or for the compounds wherein R.sub.1 and R.sub.2 are both not H, a quaternary ammonium salt thereof with a C.sub.1-4 alkyl halide have valuable antiviral activity, e.g., for treatment of infections caused by a herpes virus.式为##STR1##的化合物,其中R为H,卤素,C.sub.1-4烷基,C.sub.1-4烷氧基,3,4-亚甲二氧基,CF.sub.3,NO.sub.2,NH.sub.2,N(C.sub.1-4烷基).sub.2,CN,OH,--S(C.sub.1-4烷基)或--SO.sub.2(C.sub.1-4烷基);R.sub.1和R.sub.2分别独立为H或C.sub.1-4烷基,或与连接的N原子一起为吗啡啉,哌啶,吡咯啉,哌嗪,或N--C.sub.1-4-烷基哌嗪;当R为卤素,C.sub.1-4烷基或C.sub.1-4烷氧基时,x为0-3,否则为0或1;或其与酸的药学上可接受的盐,对于R.sub.1和R.sub.2均不为H的化合物,其与C.sub.1-4烷基卤化物的季铵盐具有有价值的抗病毒活性,例如,用于治疗由疱疹病毒引起的感染。
表征谱图
-
氢谱1HNMR
-
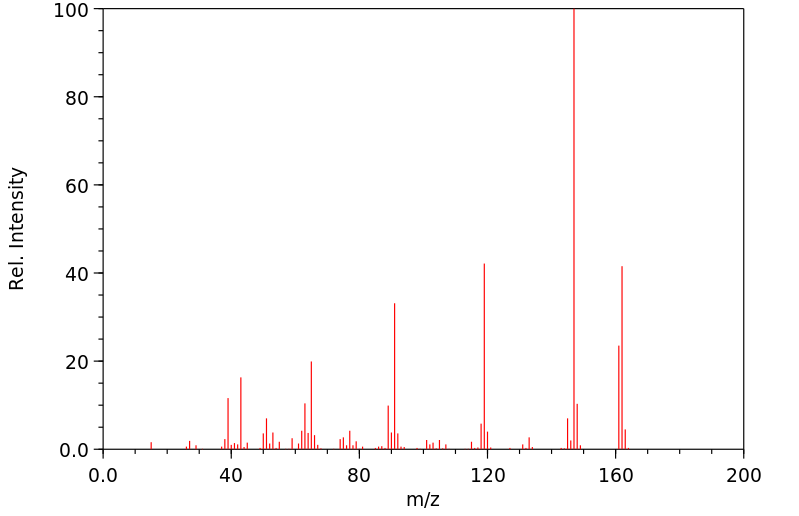
质谱MS
-
碳谱13CNMR
-
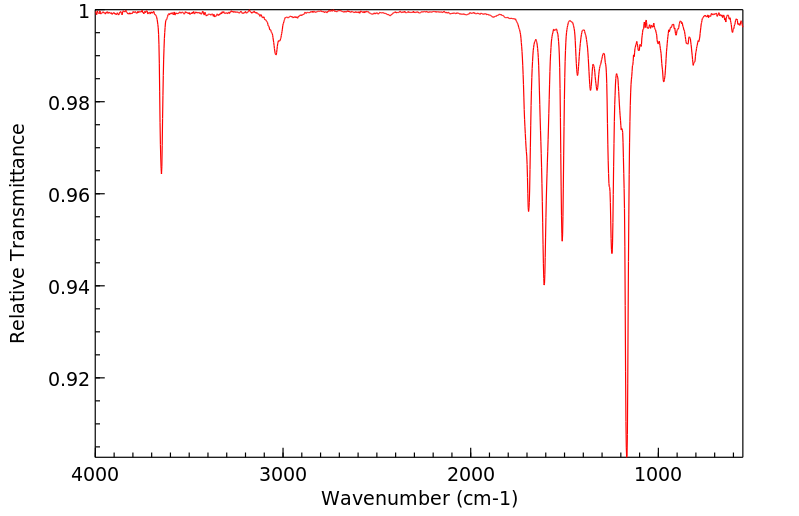
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E)-3-(4-(叔丁基)苯基)丙烯酸乙酯
(E)-3-(2-(三氟甲基)苯基)丙烯酸乙酯
(E)-3-(2,4-二甲氧基苯基)丙烯酸乙酯
(2E)-N-[2-(3-羟基-2-氧代-2,3-二氢-1H-吲哚-3-基)乙基]-3-苯基丙-2-烯酰胺
黄金树苷
鲁索曲波帕
香豆酸肉桂酯
香豆酰多巴胺
香草醛缩丙酮
顺式邻羟基肉桂酸
顺式芥子酸
顺式-曲尼司特
顺式-乙基肉桂酸酯
顺式-N-阿魏酰酪胺
顺式-3,4-二甲氧基苯丙烯酸
顺式-2-((叔丁氧羰基)氨基)-3-(4-氨甲酰基-2,6-二甲苯基)丙烯酸甲酯
顺-o-羧基肉桂酸
顺-2-甲氧基肉桂酸
阿魏酸钠
阿魏酸酰胺
阿魏酸甲酯
阿魏酸甲酯
阿魏酸甲酯
阿魏酸松柏酯
阿魏酸杂质1
阿魏酸异辛酯
阿魏酸哌嗪
阿魏酸二十烷基酯
阿魏酸乙酯
阿魏酸4-O-硫酸二钠盐
阿魏酸-D3
阿魏酸
阿魏酸
阿魏酰酪胺
间羟基肉桂酸
间羟基肉桂酸
间硝基肉桂酸
间甲基肉桂酸
间甲基反式肉桂酸甲酯
间氯肉桂酸
间三氟甲氧基肉桂酸甲酯
间-香豆酸
间-(三氟甲基)-肉桂酸
锂(E)-2-溴-3-苯基丙烯酸酯
钠二乙基2-[(氧代氨基)-苯基亚甲基]丙二酸酯盐
酪氨酸磷酸化抑制剂AG 556
酪氨酸磷酸化抑制剂AG 527
酪氨酸磷酸化抑制剂AG 490
酪氨酸磷酸化抑制剂A46
酪氨酸磷酸化抑制剂 AG 30