恩比兴 | 55-86-7
中文名称
恩比兴
中文别名
N-甲基-N-(2-氯乙基)-2-氯乙胺盐酸盐;二氯甲基二乙胺盐酸盐;盐酸氮芥;2-氯-N-(2-氯乙基)-N-甲基乙胺盐酸盐;氮芥盐酸盐
英文名称
mechlorethamine hydrochloride
英文别名
2-chloro-N-(2-chloroethyl)-N-methylethanamine hydrochloride;methylamine hydrochloride;bis-(β-chloroethyl)methylamine hydrochloride;N,N-bis(2-chloroethyl)-N-methylamine hydrochloride;N,N-di-(2-chloroethyl)-N-methylamine hydrochloride;N-methyl-N,N-bis-(2-chloroethyl)-ammonium chloride;1,5-Dichloro-3-methyl-3-azapentane, Hydrochloride;2,2'-dichloro-N-methyl-diethylamine hydrochoride;2,2'-dichloro-N-methyldiethylamine hydrochloride;N,N-bis(2-chloroethyl)methylamine hydrochloride;N,N-di(2-chloroethyl)methylamine hydrochloride;N-methyl bis(2-chloroethyl)amine hydrochloride;N,N-Bis(2-chlorethyl)-N-methylammoniumchlorid;bis(2-chloroethyl) methyl amine hydrochloride;bis(chloroethyl)-N-methylamine hydrochloride;methyl-bis(2-chloroethyl)amine hydrochloride;bis(2-chloroethyl)methylammonium chloride;nitrogen mustard hydrochloride;chlormethine hydrochloride;2-chloro-N-(2-chloroethyl)-N-methylethylamine hydrochloride;2-chloro-N-(2-chloroethyl)-N-methylethan-1-amine hydrochloride;2-chloro-N-(2-chloroethyl)-N-methylethanamine;hydron;chloride
CAS
55-86-7
化学式
C5H11Cl2N*ClH
mdl
MFCD00012517
分子量
192.516
InChiKey
QZIQJVCYUQZDIR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:108-111 °C(lit.)
-
沸点:315.95°C (rough estimate)
-
密度:1.4424 (rough estimate)
-
溶解度:易溶于水
-
物理描述:Nitrogen mustard hydrochloride appears as white to off-white crystals or powder with a fishy odor. Initial pH (2% aqueous solution) 3.0-4.0. (NTP, 1992)
-
颜色/状态:Hygroscopic leaflets from acetone or chloroform
-
稳定性/保质期:
Storage and shelf-life of unopened container: Two years when stored at 2-15 °C.
-
解离常数:pKa = 6.43 at 25 °C (conjugate acid)
计算性质
-
辛醇/水分配系数(LogP):1.52
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:3.2
-
氢给体数:1
-
氢受体数:1
ADMET
代谢
Following its in vivo admin, mechlorethamine or its hydrochloride is probably converted into ethyleneimmonium ion which reacts with guanine residues in /either the same or/ adjacent strands of DNA as well as with SH groups.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
致癌性分类:1)人类证据:有限;2)动物证据:充分。对人类致癌风险的总体评估为2A组:该物质很可能对人类致癌。/美洛昔芬;来自表格/
Classification of carcinogenicity: 1) evidence in humans: limited; 2) evidence in animals: sufficient. Overall summary evaluation of carcinogenic risk to humans is Group 2A: The agent is probably carcinogenic to humans. /Mechlorethamine; From table/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Nitrogen Mustard Hydrochloride: reasonably anticipated to be a human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
◉ 母乳喂养期间使用总结:大多数资料认为,接受抗癌治疗的母亲不应哺乳,尤其是使用甲氯乙胺等烷化剂。由于哺乳婴儿可能会出现严重的不良反应,接受甲氯乙胺治疗的母亲在甲氯乙胺治疗期间,包括局部应用时,不应哺乳。
◉ 对哺乳婴儿的影响:截至修订日期,未找到相关的已发布信息。
◉ 对泌乳和母乳的影响:截至修订日期,未找到相关的已发布信息。
◉ Summary of Use during Lactation:Most sources consider that mothers receiving antineoplastic therapy should not breastfeed, especially with alkylating agents such as mechlorethamine. Because of the potential for serious adverse reactions in the breastfed infant, breastfeeding from mechlorethamine mothers should not breastfed during therapy with mechlorethamine therapy, including topical application.
◉ Effects in Breastfed Infants:Relevant published information was not found as of the revision date.
◉ Effects on Lactation and Breastmilk:Relevant published information was not found as of the revision date.
来源:Drugs and Lactation Database (LactMed)
毒理性
Morphine and pethidine hydrochloride significantly potentiated mechlorethamine hydrochloride toxicity. The potentiation seems dose related. Morphine converted sublethal doses of mechlorethamine to maximal LD's.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
In HT29 human colon carcinoma cells, amphotericin b (approx 120 ug/ml) increased uptake of mechlorethamine hydrochloride due to increases in vmax without change in km.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Following IV injection, the drug undergoes rapid chemical transformation and unchanged mechlorethamine is undetectable in the blood within a few minutes. Less than 0.01% of an IV dose is excreted unchanged in the urine.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Mice given 35 mg/kg body wt mechlorethamine hydrochloride iv and examined by autoradiography had significant levels of compound in brain, spinal cord, lung and submaxillary glands.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
美克洛塞在经腔内给药后吸收不完全,这可能是由于体液迅速使其失效。
Mechlorethamine is incompletely absorbed following intracavitary administration, probably because of rapid deactivation by body fluids.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:6.1(a)
-
危险品标志:T+
-
安全说明:S36/37/39,S45,S53
-
危险类别码:R42/43,R45,R28,R34,R46
-
WGK Germany:3
-
海关编码:2921199090
-
危险品运输编号:UN 2811 6.1/PG 2
-
危险类别:6.1(a)
-
RTECS号:IA2100000
-
包装等级:II
-
储存条件:库房应保持通风、低温和干燥,并与食品原料分开存放。
SDS
| Name: | Mechlorethamine hydrochloride 98% Material Safety Data Sheet |
| Synonym: | N-Methylbis(2-chloroethyl)amine hydrochlorid |
| CAS: | 55-86-7 |
Synonym:N-Methylbis(2-chloroethyl)amine hydrochlorid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 55-86-7 | Mechlorethamine hydrochloride, 98% | 200-246-0 |
Risk Phrases: 26/27/28 33
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Very toxic by inhalation, in contact with skin and if swallowed.
Danger of cummulative effects.Cancer suspect agent.Hygroscopic.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower lids.
Skin:
Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. Allow the victim to rinse his mouth and then to drink 2-4 cupfuls of water, and seek medical advice.
Inhalation:
Remove from exposure to fresh air immediately.
Notes to Physician:
Treat symptomatically.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Autoignition Temperature: Not available.
Flash Point: Not available.
NFPA Rating: Not published.
Explosion Limits, Lower: Not available.
Upper: Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Not available.
Storage:
Poison room locked.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Wear a NIOSH/MSHA-approved (or equivalent) full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Not available.
Appearance: beige
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Vapor Density: Not available.
Evaporation Rate: Not available.
Viscosity: Not available.
Boiling Point: @ 760.00mm Hg
Freezing/Melting Point: 108.00 - 110.00 deg C
Decomposition Temperature: Not available.
Solubility: soluble
Specific Gravity/Density: Not available.
Molecular Formula: C5H11Cl2N.HCl
Molecular Weight: 192.52
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong oxidizing agents - moisture.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 55-86-7: IA2100000 LD50/LC50:
CAS# 55-86-7: Oral, mouse: LD50 = 20 mg/kg; Oral, rabbit: LD50 = 12500 ug/kg; Oral, rat: LD50 = 10 mg/kg.
Carcinogenicity:
Mechlorethamine hydrochloride, 98% - California: carcinogen NTP: Suspect carcinogen OSHA: Possible Select carcinogen See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
For further information, contact Fisher Scientific.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
CDG/CPL
IMO
Shipping Name: TEAR GAS SUBSTANCE, SOLID, N.O.S.
Hazard Class: 6.1
UN Number: 1693
Packing Group: II
IATA
Shipping Name: TEAR GAS SUBSTANCE, SOLID, N.O.S.*
Hazard Class: 6.1
UN Number: 1693
Packing Group: II
RID/ADR
Shipping Name: TEAR GAS SUBSTANCE, SOLID, N.O.S.
Dangerous Goods Code: 6.1(25B)
UN Number: 1693
Canadian TDG
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T+
Risk Phrases:
R 26/27/28 Very toxic by inhalation, in contact with
skin and if swallowed.
R 33 Danger of cummulative effects.
Safety Phrases:
S 22 Do not inhale dust.
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 55-86-7:
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
WHMIS: Not available.
CAS# 55-86-7 is not listed on Canada's Ingredient Disclosure List.
Exposure Limits
US FEDERAL
TSCA
CAS# 55-86-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
抗肿瘤药:恩比兴
恩比兴是最早用于临床治疗肿瘤的药物之一,可以代表其他烷化剂抗肿瘤药物。尽管其衍生物在临床上仍有重要地位,但与其他药物相比,它对肿瘤细胞和正常细胞的作用差异不大,选择性差,因此毒性也相对较大。
进入人体后,恩比兴在体内水解释放氯离子,成为游离基,并通过分子内成环作用形成高度活泼的乙烯亚胺离子。这些活性物质能够与脱氧核糖核酸发生烷化反应,进而影响核酸代谢、抑制细胞有丝分裂,具有较强的细胞毒性。
特点- 作用短暂但效果持久:恩比兴进入体内后快速解离,血浆中药物在0.5~1分钟内即消失,然而其治疗效果可以持续较长时间。
- 非选择性分布:药物在体内的分布没有明显偏好,尤其是脑内的分布较少。少量原形药物会通过尿液排出。
恩比兴在中国被用于多种恶性肿瘤的治疗,包括:
- 恶性淋巴瘤(霍奇金病、淋巴肉瘤等)
- 肺癌
- 对未分化癌有较好的疗效
- 配合其他药物使用效果更好
- 可用于治疗慢性粒细胞白血病、慢性淋巴细胞白血病、乳腺癌、卵巢癌及绒毛膜上皮癌等
此外,恩比兴通过腹主动脉阻断(半身阻断)和大剂量静脉注射的方法在头颈部癌症(如鼻咽癌)的治疗中显示出良好效果。局部使用其乙醇或二甲亚砜溶液可治疗牛皮癣、白癜风及蕈样霉菌病等皮肤病。
生物活性Mechlorethamine是烷化剂的基本形态,通过与DNA结合,交联双链并阻止细胞复制来发挥作用。
靶点- DNA合成
在无氧条件下,Mechlorethamine对大鼠肝细胞的毒性较小,并且在需氧条件下引起的脂质过氧化较少。兔子气管原代培养中,Mechlorethamine显著抑制了细胞生长并导致细胞骨架蛋白重排相关的分离。此外,它还引发了早期脂质过氧化和细胞膜损伤,这与谷胱甘肽含量增加相关的抗氧化酶活性显著提升有关。
体内研究- 减少白血球数量:Mechlorethamine (1.5毫克/千克)通过静脉注射能减少兔子体内的平均白血球数量。
- 皮肤溃疡作用:在小鼠身上,Mechlorethamine (0.005毫克-0.5毫克, i.d.) 造成了剂量依赖性皮肤溃疡。立即给予等渗或高渗硫代硫酸钠可以显著降低溃疡面积和持续时间。
口服 - 大鼠 LD50: 10 毫克/公斤; 口服 - 小鼠 LD50:20 毫克/公斤
物理化学性质与安全性- 可燃危险特性:燃烧时会产生有毒氮氧化物和氯化物烟雾。
- 药物副作用:恶心、呕吐、白血球和血小板数量减少。
反应信息
-
作为反应物:描述:参考文献:名称:肽键形成有机催化剂的合理设计摘要:酰胺键普遍存在于肽、蛋白质、药物和聚合物中。酰胺键的形成是一个相对简单的过程:由于有效偶联剂的可用性,可以相对容易地合成酰胺键。然而,对于不需要过量试剂的方法存在实质性需求。缩合氨基酸的催化剂可以通过减少肽合成过程中产生的大量废物产生重要影响。我们描述了一种仿生催化剂的合理设计,该催化剂可以有效地偶联具有标准保护基团的氨基酸。催化剂设计结合了从酶、肽生物合成和有机催化剂中吸取的经验教训。在优化条件下,5 mol% 的催化剂可以有效地偶联 Fmoc 氨基酸,而不会发生显着的外消旋化。显着地,我们证明该催化剂可用于在固相上合成寡肽。这个结果很重要,因为它说明了催化剂在具有大量酰胺键的基材上发挥作用的潜力,这可能会抑制氢键催化剂。DOI:10.1021/jacs.9b07742
-
作为产物:描述:参考文献:名称:‘3+1' Mixed-Ligand Oxotechnetium(V) Complexes with Affinity for Melanoma: Synthesis and Evaluation in Vitro and in Vivo摘要:**'3+1'型杂配位体[Tc-99m]氧肟technetium复合物的合成及其对黑色素瘤的靶向性研究** 在一步锅法反应中,成功合成了一种对黑色素瘤具有亲和力的'3+1'型杂配位体 [Tc-99m]oxotechnetium 复合物。通过将 technetium-99m 与 N-R(3-氮杂戊烷-1,5-二硫醇)[R = Me, Pr, Bn, Et2N(CH2)2] 和 N-(2-双烷基氨基)乙烷硫醇 [烷基 = X = Et, Bu, 吗啉基] 的混合物中的 technetium-99m 复合,并使用 Sn²+ 作为还原剂,最终形成了'3+1'型杂配位体 technetium-99m 复合物 [TcO(SN(R)S)(SNX2)],其放射化学产率高达 60-98% 。 体外实验中,利用 B16 小鼠黑色素瘤细胞进行的摄取研究表明,化合物 1 [R = Me, X = Et] 在60 分钟孵育后的肿瘤细胞积聚量为 40%,而化合物 2 [R = Me, X = Bu] 则达到了 69% 的较高积聚量。在 C57B16/B16 小鼠黑色素瘤模型中,对化合物 1-6 进行的体内评估显示,这些化合物均表现出肿瘤定位能力。其中,化合物 2 在注射后60 分钟内达到最高的积聚量,为 5% ID/g 。 进一步的体内研究表明,化合物 2 在1 小时时表现出较低的血液活性,并且其黑色素瘤/脾脏(4.3)和黑色素瘤/肺(1.9)比值显著升高。这些结果表明,小巧的 technetium-99m 复合物有望成为潜在的黑色素瘤成像剂。DOI:10.1021/jm000050e
-
作为试剂:描述:甲酸 、 [NH2,CONH2-15N2]-5-amino-4-imidazolecarboxamide 在 恩比兴 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 4.0h, 以92%的产率得到[N1,N3,15N2]hypoxanthine参考文献:名称:15N-多标记腺嘌呤和鸟嘌呤核苷。[1,3,NH(2)-(15)N(3)]-和[2-(13)C-1,3,NH(2)-(15)N(3)]标记的腺苷的合成,鸟苷,2'-脱氧腺苷和2'-脱氧鸟苷。摘要:我们报告到以下具体(15)N-和(13)C多标记核苷的高产途径:[1,3,NH(2)-(15)N(3)]-和[2-(13 )C-1,3,NH(2)-(15)N(3)]-腺苷;[1,3,NH(2)-(15)N(3)]-和[2-(13)C-1,3,NH(2)-(15)N(3)]-鸟苷;[1,3,NH(2)-(15)N(3)]-和[2-(13)C-1,3,NH(2)-(15)N(3)]-2'-脱氧腺苷; [1,3,NH(2)-(15)N(3)]-和[2-(13)C-1,3,NH(2)-(15)N(3)]-2'-脱氧鸟苷。在每组中,(13)C2原子充当“标签”,使(15)N1和(15)N3原子与RNA或DNA片段的(15)N NMR中的未标记形式毫无区别。腺嘌呤和鸟嘌呤核苷的这种合成策略的关键中间体是[NH(2),CONH(2)-(15)N(2)]-5-氨基-4-咪唑甲酰胺。[2-(13)C]-标DOI:10.1021/jo982372k
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] COMPOUNDS AND COMPOSITIONS COMPRISING CDK INHIBITORS AND METHODS FOR THE TREATMENT OF CANCER<br/>[FR] COMPOSÉS ET COMPOSITIONS COMPRENANT DES INHIBITEURS DES CDK ET MÉTHODES DE TRAITEMENT DU CANCER申请人:UNIV GEORGIA STATE RES FOUND公开号:WO2010129858A1公开(公告)日:2010-11-11Disclosed herein are compounds suitable for use as antitumor agents, methods for treating cancer wherein the disclosed compounds are used in making a medicament for the treatment of cancer, methods for treating a tumor comprising, administering to a subject a composition comprising one or more of the disclosed cytotoxic agents, and methods for preparing the disclosed antitumor agents.
-
SULFOXIMINE SUBSTITUTED QUINAZOLINES FOR PHARMACEUTICAL COMPOSITIONS申请人:BLUM Andreas公开号:US20140135309A1公开(公告)日:2014-05-15This invention relates to novel sulfoximine substituted quinazoline derivatives of formula I wherein Ar, R 1 and R 2 are as defined herein, and their use as MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
[EN] SULFOXIMINE SUBSTITUTED QUINAZOLINES FOR PHARMACEUTICAL COMPOSITIONS<br/>[FR] QUINAZOLINES SUBSTITUÉES PAR SULFOXIMINE POUR COMPOSITIONS PHARMACEUTIQUES申请人:BOEHRINGER INGELHEIM INT公开号:WO2014072244A1公开(公告)日:2014-05-15This invention relates to novel sulfoximine substituted quinazoline derivatives of formula (I), wherein Ar, R1 and R2 are as defined in the description and claims, and their use as MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
Thieno-pyrimidine compounds having fungicidal activity
表征谱图
-
氢谱1HNMR
-
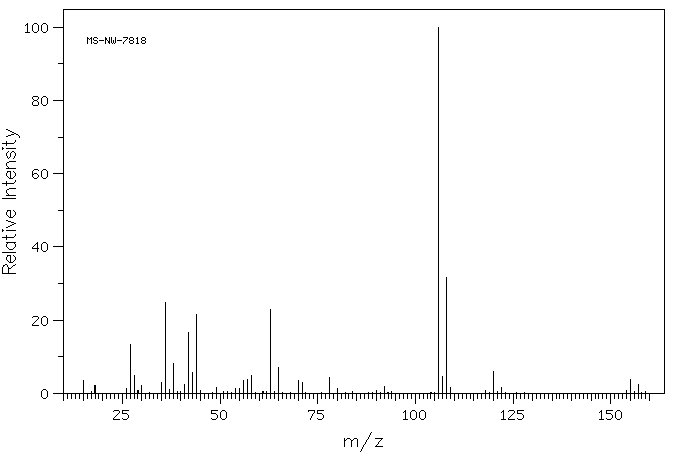
质谱MS
-
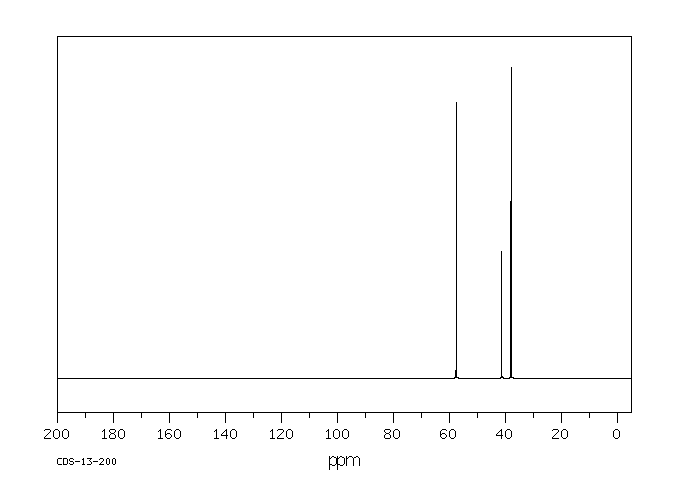
碳谱13CNMR
-
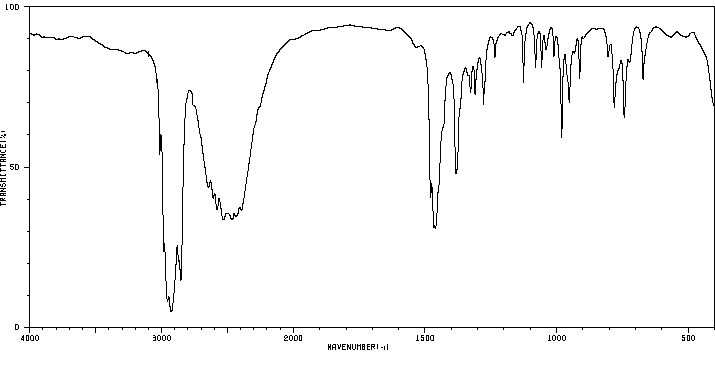
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷