1,2-二哌啶基乙烷 | 1932-04-3
中文名称
1,2-二哌啶基乙烷
中文别名
1,2-二哌啶乙烷;1,2-二哌啶-1-基乙烷
英文名称
1,2-dipiperidinoethane
英文别名
1-(2-piperidin-1-ylethyl)piperidine
CAS
1932-04-3
化学式
C12H24N2
mdl
MFCD00014644
分子量
196.336
InChiKey
UYMQPNRUQXPLCY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-1--0.5 °C
-
沸点:265 °C
-
密度:0.91
-
闪点:110 °C
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:6.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2933399090
-
WGK Germany:3
-
储存条件:密封储存,应存放在阴凉、干燥的仓库中。
SDS
| Name: | 1 2-Dipiperidinoethane 98% Material Safety Data Sheet |
| Synonym: | 1,1'-(1,2-Ethanediyl)bispiperidin |
| CAS: | 1932-04-3 |
Synonym:1,1'-(1,2-Ethanediyl)bispiperidin
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1932-04-3 | 1,2-Dipiperidinoethane | 98 | 217-692-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Hygroscopic (absorbs moisture from the air).The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
Causes gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Do NOT get water inside containers. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Do not allow contact with water. Keep from contact with moist air and steam.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1932-04-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 265 deg C @ 760.00mmHg
Freezing/Melting Point: 1 deg C
Autoignition Temperature: Not available.
Flash Point: 110 deg C ( 230.00 deg F)
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9160g/cm3
Molecular Formula: C12H24N2
Molecular Weight: 196.33
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, moisture, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1932-04-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,2-Dipiperidinoethane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1932-04-3: No information available.
Canada
CAS# 1932-04-3 is listed on Canada's NDSL List.
CAS# 1932-04-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1932-04-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-甲基哌啶 N-Methylpiperidine 626-67-5 C6H13N 99.1759 1-(2-氯乙基)哌啶 2-(piperidin-4-yl)ethyl chloride 1932-03-2 C7H14ClN 147.648 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-甲基哌啶 N-Methylpiperidine 626-67-5 C6H13N 99.1759 —— 1,2-Di-(N-piperidyl)ethan-bis-N-oxid 68601-52-5 C12H24N2O2 228.335
反应信息
-
作为反应物:描述:1,2-二哌啶基乙烷 在 5 wt% Pd/C 、 potassium tert-butylate 、 氢气 、 1-butyl-3-methylimidazolium Tetrafluoroborate 作用下, 160.0 ℃ 、6.0 MPa 条件下, 反应 8.0h, 生成 N-甲基哌啶参考文献:名称:离子液体-Pd/C体系催化的环胺和CO2/H2选择性合成甲酰胺、1,2-双(N-杂环)乙烷和甲胺。摘要:用胺和 H2 还原 CO2 通常在不同的催化剂上产生 N-甲酰化或 N-甲基化化合物。在此,我们报道了甲酰胺、1,2-双(N-杂环)乙烷和甲胺的选择性合成,这是在离子液体(IL,例如,1-丁基-3-甲基咪唑鎓四氟硼酸盐,[BMIm][ BF4])-Pd/C 催化体系。通过简单地改变反应温度,可以分别以高产率选择性地生产甲酰胺和甲胺。有趣的是,1,2-双(N-杂环)乙烷也可以通过形成的甲酰胺的 McMurry 反应与随后的氢化反应来获得。发现[BMIm][BF4]可以与甲酰胺反应形成[BMIm]+-甲酰胺加合物;因此与 Pd/C 结合,它可以在 H2 存在下催化甲酰胺的 McMurry 偶联,得到 1,2-双(N-杂环)乙烷。此外,Pd/C-[BMIm][BF4]可以进一步催化1,2-双(N-杂环)乙烷氢解得到甲胺。[BMIm][BF4]-Pd/C 对广泛的底物范围具有耐受性,以中等至高产率提供相应的甲酰胺、1DOI:10.1039/c9sc03242h
-
作为产物:描述:哌啶-1-甲醛 在 N-甲基哌啶 、 5 wt% Pd/C 、 氢气 、 1-butyl-3-methylimidazolium Tetrafluoroborate 作用下, 160.0 ℃ 、6.0 MPa 条件下, 反应 8.0h, 生成 1,2-二哌啶基乙烷参考文献:名称:离子液体-Pd/C体系催化的环胺和CO2/H2选择性合成甲酰胺、1,2-双(N-杂环)乙烷和甲胺。摘要:用胺和 H2 还原 CO2 通常在不同的催化剂上产生 N-甲酰化或 N-甲基化化合物。在此,我们报道了甲酰胺、1,2-双(N-杂环)乙烷和甲胺的选择性合成,这是在离子液体(IL,例如,1-丁基-3-甲基咪唑鎓四氟硼酸盐,[BMIm][ BF4])-Pd/C 催化体系。通过简单地改变反应温度,可以分别以高产率选择性地生产甲酰胺和甲胺。有趣的是,1,2-双(N-杂环)乙烷也可以通过形成的甲酰胺的 McMurry 反应与随后的氢化反应来获得。发现[BMIm][BF4]可以与甲酰胺反应形成[BMIm]+-甲酰胺加合物;因此与 Pd/C 结合,它可以在 H2 存在下催化甲酰胺的 McMurry 偶联,得到 1,2-双(N-杂环)乙烷。此外,Pd/C-[BMIm][BF4]可以进一步催化1,2-双(N-杂环)乙烷氢解得到甲胺。[BMIm][BF4]-Pd/C 对广泛的底物范围具有耐受性,以中等至高产率提供相应的甲酰胺、1DOI:10.1039/c9sc03242h
-
作为试剂:参考文献:名称:通过关键的中间体α-苯基膦酰基锂硼烷络合物合成新的手性来源(1R,2S)-1-苯基膦烷-2-羧酸:α-单膦烷基烷基硼烷络合物的构型稳定性和X射线晶体结构。摘要:建立了对映体纯的(1R,2S)-1-苯基膦环烷-2-羧酸(1)(脯氨酸的磷类似物)的合成途径。关键步骤是通过使用sBuLi / 1,2-二哌啶基乙烷(DPE)进行1-苯基膦烷硼烷络合物3的去质子化-羧化。在多种胺存在下,通过对1-苯基-2-三甲基锡烷基膦烷硼烷络合物7的两个非对映异构体进行硫代去锡烷基化-羧化反应,研究了关键中间体胺配位的α-膦烷基烷基硼烷络合物4的构型稳定性,结果发现,即使在-100摄氏度下,4仍然具有构型不稳定。分离出了关键中间体,即DPE配位的反式-1-苯基-2-膦酰基硼烷硼烷络合物9,并通过X射线晶体结构分析鉴定其结构。这是为α-单膦烷基烷基硼烷络合物确定的第一个X射线晶体结构。值得注意的是,烷基锂络合物在锂中心略呈金字塔形的环境中呈单体和三配位,并且已证实存在Li-C键(2.170 A)。此外,(1)H-(7)Li HOESY和(6)Li NMR分析表明,溶液中9DOI:10.1002/chem.200400631
文献信息
-
Processes for preparing functionahzed polymers, related functionalizing compound and preparation thereof申请人:Bridgestone Corporation公开号:US10730961B2公开(公告)日:2020-08-04Disclosed herein are processes for preparing functionalized polymers, functionalizing compounds useful in the processes and processes for preparing the functionalizing compound. The processes for preparing a functionalized polymer include reaction of a functionalizing compound (prepared from the reaction of an alkoxysilane compound of formula (I) with a polyol of formula (II)) with a reactive conjugated diene monomer-containing polymer, thereby producing a polymer end functionalized with the functionalizing compound. In certain embodiments, the functionalizing compound has a formula according to formula (III), (IV), or (V).公开了用于制备官能化聚合物的过程、在过程中有用的官能化化合物以及制备官能化化合物的过程。制备官能化聚合物的过程包括使官能化化合物(由公式(I)的烷氧基硅烷化合物与公式(II)的多羟基醇反应制得)与包含反应性共轭二烯单体聚合物反应,从而产生以官能化化合物端基官能化的聚合物。在某些实施方式中,官能化化合物的公式符合公式(III)、(IV)或(V)。
-
Synthesis of Al-MTW with low Si/Al ratios by combining organic and inorganic structure directing agents
-
Bifunctional Acid-Base Ionic Liquid Organocatalysts with a Controlled Distance Between Acid and Base Sites作者:Mercedes Boronat、Maria J. Climent、Avelino Corma、Sara Iborra、Raquel Montón、Maria J. SabaterDOI:10.1002/chem.200901519日期:2010.1.25Bifunctional acid–base ionic liquid organocatalysts with different distances between the two sites have been synthesised, and their activity for the Knoevenagel condensation has been tested. As has been found to be the case with enzymes, the distance between the acidic and basic sites determines the activity of the bifunctional organocatalyst, and at the optimal distance the reaction rate increases
-
Visible-light photo-catalytic C–C bond cleavages: preparations of N,N-dialkylformamides from 1,2-vicinal diamines作者:Yaohong Zhao、Shunyou Cai、Jing Li、David Zhigang WangDOI:10.1016/j.tet.2013.07.056日期:2013.9A range of 1,2-vicinal diamines were smoothly converted into N,N-dialkylformamides under the synergistic actions of Ru(bpy)3Cl2 photo-catalyst, 45 W household lighting bulb, and Cs2CO3 basic additive under very mild reaction conditions. The process involves visible light-enabled photo-catalytic cleavage of C–C bond as the strategic event.在非常温和的条件下,Ru(bpy)3 Cl 2光催化剂,45 W家用电灯泡和Cs 2 CO 3碱性添加剂的协同作用下,将一系列1,2-邻位二胺顺利转化为N,N-二烷基甲酰胺反应条件。该过程涉及可见光激活的C–C键的光催化裂解,这是战略性事件。
-
Aminosilyl-Substituted Diarylethene Compounds for Anionic Polymerisation申请人:Trinseo Europe GmbH公开号:US20170204119A1公开(公告)日:2017-07-20The invention related to novel compounds useful as modifying monomers and precursors for polymerization initiators. The invention further relates to a method of making the polymerization initiators and resulting polymers. The invention also relates to polymer compositions comprising the polymer of the invention and further components such as extender oils, fillers, vulcanizing agents etc., and to corresponding vulcanized polymer compositions and articles comprising vulcanized parts made from the vulcanized polymer composition.
表征谱图
-
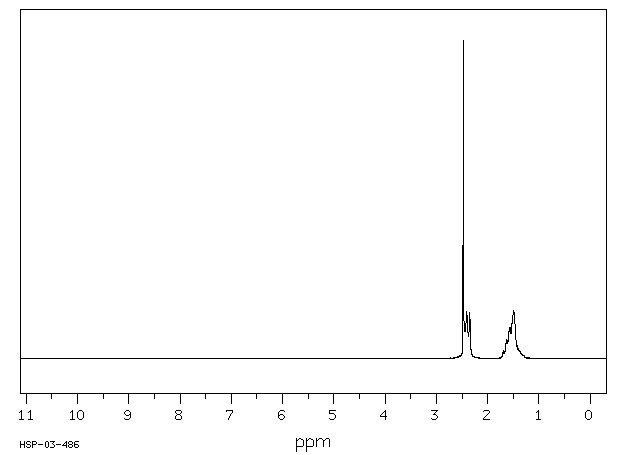
氢谱1HNMR
-
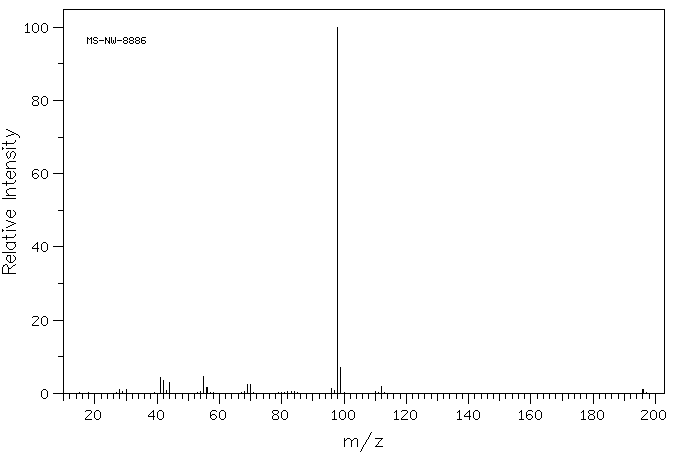
质谱MS
-
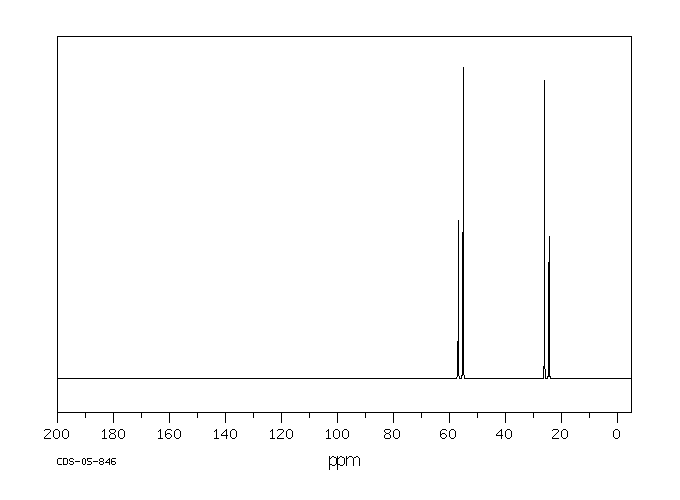
碳谱13CNMR
-
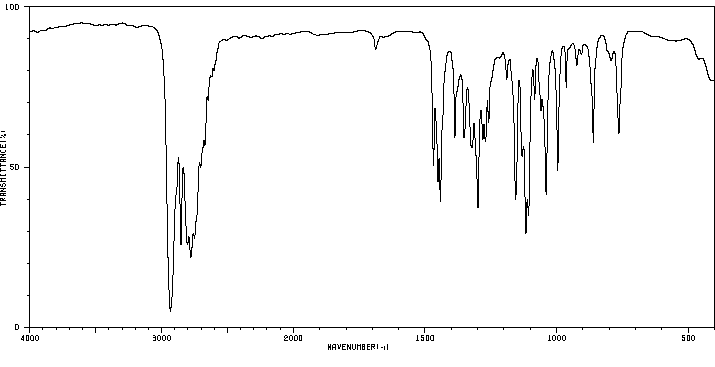
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺