1-溴-1-环己烯E | 2044-08-8
中文名称
1-溴-1-环己烯E
中文别名
1-溴-1-环己烯;1-溴环己-1-烯
英文名称
1-bromocyclohex-1-ene
英文别名
1-bromocyclohexene;1-bromo-1-cyclohexene;cyclohexenyl bromide;1-cyclohexenyl bromide;bromocyclohexene
CAS
2044-08-8
化学式
C6H9Br
mdl
——
分子量
161.041
InChiKey
QBUMXSSCYUMVAW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:155.42°C (estimate)
-
密度:1.3901
-
保留指数:975;975
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903890090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:-20°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1-Bromo-1-cyclohexene
Synonyms: 1-Bromocyclohex-1-ene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Bromo-1-cyclohexene
CAS number: 2044-08-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, under −20◦C.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C6H9Br
Molecular weight: 161.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1-Bromo-1-cyclohexene
Synonyms: 1-Bromocyclohex-1-ene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1-Bromo-1-cyclohexene
CAS number: 2044-08-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, under −20◦C.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C6H9Br
Molecular weight: 161.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-溴环己-2-烯-1-酮 3-bromo-2-cyclohexen-1-one 56671-81-9 C6H7BrO 175.025
反应信息
-
作为反应物:描述:参考文献:名称:Cornubert; Rio; Senechal, Bulletin de la Societe Chimique de France, 1955, p. 46,50摘要:DOI:
-
作为产物:描述:1-氯环己烯 生成 1-溴-1-环己烯E参考文献:名称:1-卤代环己烯和甲基取代的1-卤代环己烯与叔丁醇钾的反应摘要:1-卤代环己烯和1-卤代-4-甲基环己烯与叔丁醇钾(t-BuOK)在二甲亚砜和四氢呋喃中的反应已通过三种竞争的脱卤化氢机理发生。它们是:工作在C脱卤化氢1 C 6键,得到cyclohexyne; 工作在C脱卤化氢1 C 6键合得到1,2-环己二烯:和质子重排成相应的3-卤代环己烯,然后β-消除成1,3-环己二烯。高度应变的环己炔和1,2-环己二烯中间体与t-BuOK反应生成1-叔丁氧基环己烯,在DMSO中的产率为5–20%,在THF中的产率为60%。与取代反应竞争的是1,2-环己二烯二聚为三环[6.4.0.0 2,7 ] -dodeca - 2,12 -diene,以及1,2-和1的1,2-和1,4-环加成,3-环己二烯。DOI:10.1016/0040-4020(72)88141-9
-
作为试剂:描述:2-甲基-1,3-环戊二酮 、 丙二烯 在 1-溴-1-环己烯E 、 sodium hydride 、 三苯基膦 、 bis(dibenzylideneacetone)-palladium(0) 作用下, 以 四氢呋喃 为溶剂, 反应 72.0h, 以59%的产率得到2-<2-(cyclohex-1-ene-1-yl)prop-2-ene-1-yl>-2-methylcyclopentane-1,3-dione参考文献:名称:Gauthier, Veronique; Gazes, Bernard; Gore, Jacques, Bulletin de la Societe Chimique de France, 1996, vol. 133, # 6, p. 563 - 579摘要:DOI:
文献信息
-
Enantioselective Cross-Coupling of <i>meso</i>-Epoxides with Aryl Halides作者:Yang Zhao、Daniel J. WeixDOI:10.1021/jacs.5b01909日期:2015.3.11The first enantioselective cross-electrophile coupling of aryl bromides with meso-epoxides to form trans-β-arylcycloalkanols is presented. The reaction is catalyzed by a combination of (bpy)NiCl2 and a chiral titanocene under reducing conditions. Yields range from 57 to 99% with 78–95% enantiomeric excess. The 30 examples include a variety of functional groups (ether, ester, ketone, nitrile, ketal
-
Palladium-Catalyzed Decarboxylative γ-Olefination of 2,5-Cyclohexadiene-1-carboxylic Acid Derivatives with Vinyl Halides作者:Chi-Hao Chang、Chih-Ming ChouDOI:10.1021/acs.orglett.8b00486日期:2018.4.6This study explores a Pd-catalyzed decarboxylative Heck-type Csp3–Csp2 coupling reaction of 2,5-cyclohexadiene-1-carboxylic acid derivatives with vinyl halides to provide γ-olefination products. The olefinated 1,3-cyclohexadienes can be further oxidized to produce meta-alkylated stilbene derivatives. Additionally, the conjugated diene products can also undergo a Diels–Alder reaction to produce a bicyclo[2
-
Synthesis of Vinyl- and Aryl-Acyl Sulfonimidamides Through Pd-Catalyzed Carbonylation Using Mo(CO)<sub>6</sub>as ex situ CO Source作者:Prasad B. Wakchaure、Sanjay R. Borhade、Anja Sandström、Per I. ArvidssonDOI:10.1002/ejoc.201403148日期:2015.1We report the synthesis of N-(α,β-unsaturated acyl)-substituted sulfonimidamides through a Pd-catalyzed carbonylation protocol, employing sulfonimidamides as nucleophiles using CO gas released ex situ, and vinyl/aryl halides and triflates as reagents. The reaction is general and offers a unique class of products in moderate to good yields for a wide range of substrates and electrophiles; for example
-
One-Pot Double-Annulation Strategy for the Synthesis of Unusual Fused Bis-Heterocycles作者:Shukree Abdul-Rashed、Georgios Alachouzos、William W. Brennessel、Alison J. FrontierDOI:10.1021/acs.orglett.0c01351日期:2020.6.5A novel metal-free double-annulation cascade for the construction of unusual fused heterocyclic systems is described. This simple protocol enables the sequential assembly of two rings in one pot from two simple precursors. Acidic conditions promote the condensation and the intramolecular alkynyl Prins reaction of an enyne or arenyne alcohol with a cyclic hemiaminal to form a five-, six-, or seven-membered
-
Formation of Enehydrazine Intermediates through Coupling of Phenylhydrazines with Vinyl Halides: Entry into the Fischer Indole Synthesis作者:Fuxu Zhan、Guangxin LiangDOI:10.1002/anie.201207173日期:2013.1.21Cut to the chase: Direct formation of an enehydrazine, an intermediate in the classic Fischer indole synthesis, solves the regioselectivity problem associated with indolization. This approach not only achieves selective synthesis of indoles through proper selection of the vinyl halide, but also leads to quick construction of desoxyeseroline and esermethole, as well as the key structural motif in the
表征谱图
-
氢谱1HNMR
-
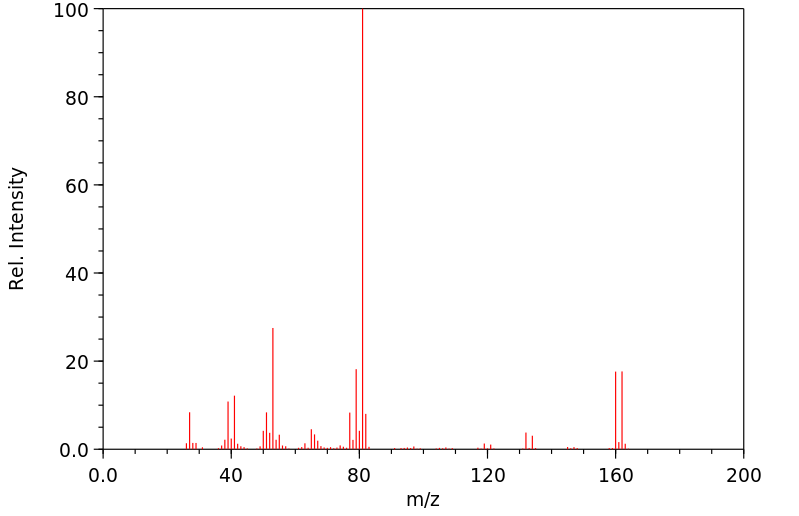
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-3-甲基-1,2,3,4-四氯-1-丁烯
顺式-1-溴-1-丙烯
顺式-1-氯-1-丁烯
顺式-1,3-二氯丙烯
顺式-1,2-二碘乙烯
顺式-1,2-二溴乙烯
顺式-1,2-二氟-1-氯乙烯
顺-氯丹
顺-九氯
顺-九氯
顺-1-溴-2-乙氧基乙烯
顺-1,2-二氯乙烯
顺-1,2,4-三氯-3-甲基-2-丁烯
顺,顺-1,2,3,4-四氯-1,3-丁二烯
除螨灵
锗烷,(1-溴-1,2-丙二烯基)三甲基-
锌,氯(三氟乙烯基)-
铜(1+),1,1,2-三氟乙烯
苯甲酸,4-[(1E)-2-[[(4-氯苯基)甲基]磺酰]乙烯基]-
苯并烯氟菌唑中间体
艾日布林-2碘
聚(乙烯-氯代三氟乙烯)
碳化镁碘化物
碘化乙烯
硫丹醇
硅烷,二氯(2-氯乙烯基)甲基-
硅烷,[2-(碘亚甲基)己基]三甲基-,(Z)-
甲碘乙烯
甲氧基全氟丁烷-反式-1,2-二氯乙烯1:1共沸物
甲基烯丙基溴化镁
甲基全氟-1-甲基-2-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
甲基全氟-1-丁基-1-丙烯基醚
环丙烷,1,1-二氯-2-(3,3-二氯-2-甲基-2-丙烯基)-2,3,3-三甲基-
环丙烯,1,2-二氟-
特比萘芬杂质
溴西克林
溴甲基烯酮
溴环辛四烯
溴氯丙烯
溴代三氟代乙烯
溴亚甲基环己烷
溴乙烯
溴三碘乙烯
氰尿酰氟
氯磺酸三氟乙烯基酯
氯化聚乙烯
氯乙烯与异丁基乙烯醚共聚物
氯乙烯与三氯乙烯聚合物
氯乙烯-d3