2,2-二苯基乙醇 | 1883-32-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:53-56 °C (lit.)
-
沸点:190-192 °C/12 mmHg (lit.)
-
密度:1.079
-
闪点:>230 °F
-
保留指数:1447
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:15
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
WGK Germany:3
-
海关编码:2906299090
-
危险类别:IRRITANT
-
储存条件:常温下应存放在阴凉、通风的地方。
SDS
模块 1. 化学品
1.1 产品标识符
: 2,2-二苯基乙醇
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C14H14O
分子式
: 198.26 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 53 - 56 °C - lit.
f) 沸点、初沸点和沸程
190 - 192 °C 在 16 hPa - lit.
g) 闪点
> 113.00 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2-二苯基乙酸 2,2-diphenylacetic acid 117-34-0 C14H12O2 212.248 联苯乙醛 2,2-diphenylethanal 947-91-1 C14H12O 196.249 (2-溴乙烷-1,1-二基)二苯 2,2-diphenylethyl bromide 40231-75-2 C14H13Br 261.161 二苯乙腈 Diphenylacetonitrile 86-29-3 C14H11N 193.248 二苯基乙酸甲酯 methyl 2,2-diphenylacetate 3469-00-9 C15H14O2 226.275 —— triethyl(2,2-diphenylethoxysilane) 56937-47-4 C20H28OSi 312.527 2,2-二苯乙酸乙酯 ethyl diphenylacetate 3468-99-3 C16H16O2 240.302 1,1-二苯基环氧乙烷 1,1-diphenyloxirane 882-59-7 C14H12O 196.249 —— (1-chloroethane-1,1-diyl)dibenzene 947-40-0 C14H13Cl 216.71 二苯基乙酰氯 diphenylacetic acid chloride 1871-76-7 C14H11ClO 230.694 2,2-联苯基乙酰胺 2,2-diphenylacetamide 4695-13-0 C14H13NO 211.263 3,3-二苯基丙酸 3,3-Diphenylpropionic acid 606-83-7 C15H14O2 226.275 —— benzyl 2,2-diphenylacetate 37537-23-8 C21H18O2 302.373 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (2-甲氧基-1-苯乙基)苯 2,2-diphenylethyl methyl ether 41976-80-1 C15H16O 212.291 苯基乙苯 1,1'-ethylidenebis-benzene 612-00-0 C14H14 182.265 —— 2,2-diphenylethyl acetate 6319-82-0 C16H16O2 240.302 —— 2,2-diphenyl-1-ethanol nitrate 130210-00-3 C14H13NO3 243.262 —— [2-(2,2-diphenyl-ethoxy)-ethyl]-dimethyl-amine 15518-86-2 C18H23NO 269.387 联苯乙醛 2,2-diphenylethanal 947-91-1 C14H12O 196.249 1,1-二苯基-2-氯乙烷 1,1-diphenyl-2-chloroethane 5216-46-6 C14H13Cl 216.71 (2-溴乙烷-1,1-二基)二苯 2,2-diphenylethyl bromide 40231-75-2 C14H13Br 261.161 —— 2,2-diphenylethyl iodide 178685-02-4 C14H13I 308.162 —— 2,2-diphenylethyl methanesulfonate 205390-22-3 C15H16O3S 276.356 1,1-二苯基乙硫醇 2,2-diphenylethanethiol 71351-02-5 C14H14S 214.331 —— Sulfamic acid 2,2-diphenyl-ethyl ester 106881-53-2 C14H15NO3S 277.344 3,3-二苯基丙腈 3,3-diphenyl-propionitrile 2286-54-6 C15H13N 207.275 双(2,2-二苯基乙基)乙二酯 oxalic acid bis-(2,2-diphenyl-ethyl ester) 7512-05-2 C30H26O4 450.534 —— 2,2-bis(4'-nitrophenyl)ethan-1-ol 116868-98-5 C14H12N2O5 288.26 —— 2,2-diphenylethyl acetoacetate 314775-53-6 C18H18O3 282.339 4,4-二苯基丁烷-1-醇 4,4-diphenylbutan-1-ol 56740-71-7 C16H18O 226.318 —— di-(2,2-diphenylethyl) disulfide 863911-83-5 C28H26S2 426.646 4,4-二苯基丁酸 4,4-diphenylbutanoic acid 14578-67-7 C16H16O2 240.302 —— 4-(2,2-Diphenylethoxy)-pyridine-1-oxide 80277-93-6 C19H17NO2 291.349 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:有机催化立体特异性Appel反应摘要:在此,我们报告了一种通过 P(III)/P(V) 氧化还原循环进行催化 Appel 反应的新方法,催化剂负载量极低(1-2 mol%),使用少量六氯丙酮作为卤素源,苯基硅烷作为末端还原剂。含有多种官能团的 26 种醇和 9 种环氧化物被转化为各自的氯化物和二氯化物,产率高达 97%,对映体特异性高达 >99%,对映体比率高达 >99:1。DOI:10.1021/acs.orglett.3c03463
-
作为产物:描述:参考文献:名称:Ramart; Amagat, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1924, vol. 179, p. 900摘要:DOI:
文献信息
-
Mild reduction of carboxylic acids to alcohols using cyanuric chloride and sodium borohydride作者:Massimo Falorni、Andrea Porcheddu、Maurizio TaddeiDOI:10.1016/s0040-4039(99)00734-0日期:1999.6Several carboxylic acids, including N-Boc, N-Cbz and N-Fmoc amino acids were reduced to the corresponding alcohols by activation of the carboxy function with cyanuric chloride and N-methylmorpholine followed by reduction with aqueous sodium borohydride.
-
Effect of cyclodextrin on elimination reactions作者:Luis Viola、Rita H de RossiDOI:10.1139/v99-085日期:1999.6.1
The reaction of 1-bromo-2-X-2-(Y-phenyl) ethane derivatives (1: X = Y = H; 2: X = Ph, Y = H; 3: X = H, Y = 4-Ac; 4: X = H, Y = 3-NO2; 5: X = H, Y = 4-NO2; 6: X = H, Y = 3-Me; 7: X = H, Y = 4-Me) in basic solution was studied, and in most cases, only the elimination product is formed. Only (2-bromo-1-phenylethyl)benzene, 2, yielded significant substitution product, and this yield decreased with the concentration of HO-. Addition of cyclodextrin (β-CD) diminished (about half for 0.02 M cyclodextrin concentration) the reaction rate of all substrates but 4 and 5. In the latter two cases, the rate rises. The observed rate-constant value at 0.5 M NaOH is 6.78 × 10-4 s-1 (at 40°C) and 1.80 × 10-3 s-1 (at 25°C) for 4 and 5, respectively. Under the same reaction conditions but with 0.01 M β-CD, the corresponding rates were 7.70 × 10-4 s-1 and 5.20 × 10-3 s-1. The elimination yield for 2 increased from 64 to 98% when the β-CD changed from zero to 0.02 M at 0.5 M NaHO. Also, there was an increase in the relative elimination products of 20-40% for compounds 6 and 7. The Hammet ρ values were 1.3 and 2.3 for the reaction in pure solvent and in the presence of β-cyclodextrin, indicating an increase in the negative character of the transition state for the reactions in the latter conditions. The results are interpreted in terms of the formation of an inclusion complex whose structure depends on the substrate.Key words: cyclodextrin, elimination reactions, inhibition, catalysis.
1-溴-2-X-2-(Y-苯基)乙烷衍生物(1:X = Y = H;2:X = Ph,Y = H;3:X = H,Y = 4-Ac;4:X = H,Y = 3-NO2;5:X = H,Y = 4-NO2;6:X = H,Y = 3-Me;7:X = H,Y = 4-Me)在碱性溶液中的反应进行了研究,在大多数情况下,只形成消除产物。只有(2-溴-1-苯基乙基)苯,2,产生了显著的取代产物,这种产量随着HO-浓度的减少而减少。环糊精(β-CD)的添加减少了所有底物的反应速率(对于0.02 M环糊精浓度减少了约一半),但对于4和5而言,速率却上升了。在后两种情况下,速率增加。在0.5 M NaOH下观察到的速率常数值分别为6.78 × 10-4 s-1(在40°C下)和1.80 × 10-3 s-1(在25°C下)对于4和5。在相同的反应条件下,但使用0.01 M β-CD,相应的速率分别为7.70 × 10-4 s-1和5.20 × 10-3 s-1。当β-CD从零变为0.02 M时,2的消除产量从64%增加到98%。此外,化合物6和7的相对消除产物增加了20-40%。在纯溶剂和β-环糊精存在的情况下,Hammet ρ值分别为1.3和2.3,表明在后一种条件下反应的过渡态的负性特征增加。结果根据形成取决于底物的包含复合物的结构进行解释。关键词:环糊精,消除反应,抑制,催化。 -
A New Method for Oxidation of Various Alcohols to the Corresponding Carbonyl Compounds by Using<i>N</i>-<i>t</i>-Butylbenzenesulfinimidoyl Chloride作者:Jun-ichi Matsuo、Daisuke Iida、Kazuya Tatani、Teruaki MukaiyamaDOI:10.1246/bcsj.75.223日期:2002.2Various primary and secondary alcohols were smoothly oxidized to the corresponding aldehydes and ketones by using a new oxidizing agent, N-t-butylbenzenesulfinimidoyl chloride (4a), in the coexistence of DBU or zinc oxide. The present oxidation proceeded under mild conditions via five-membered intramolecular proton-transfer of an alkyl arenesulfinimidate intermediate.
-
Silica Gel Promotes Reductions of Aldehydes and Ketones by<i>N</i>-Heterocyclic Carbene Boranes作者:Tsuyoshi Taniguchi、Dennis P. CurranDOI:10.1021/ol302010f日期:2012.9.7the reduction of aldehydes and ketones in the presence of silica gel. Primary and secondary alcohols are formed in good yields under ambient conditions. Aldehydes are selectively reduced in the presence of ketones. One, two, or even all three of the boron hydrides can be transferred. The process is attractive because all the components are stable and easy to handle and because both the reaction and
-
Facile Protocol for Catalytic Frustrated Lewis Pair Hydrogenation and Reductive Deoxygenation of Ketones and Aldehydes作者:Tayseer Mahdi、Douglas W. StephanDOI:10.1002/anie.201503087日期:2015.7.13A series of ketones and aldehydes are reduced in toluene under H2 in the presence of 5 mol % B(C6F5)3 and either cyclodextrin or molecular sieves affording a facile metal‐free protocol for reduction to alcohols. Similar treatment of aryl ketones resulted in metal‐free deoxygenation yielding aromatic hydrocarbons.
表征谱图
-
氢谱1HNMR
-
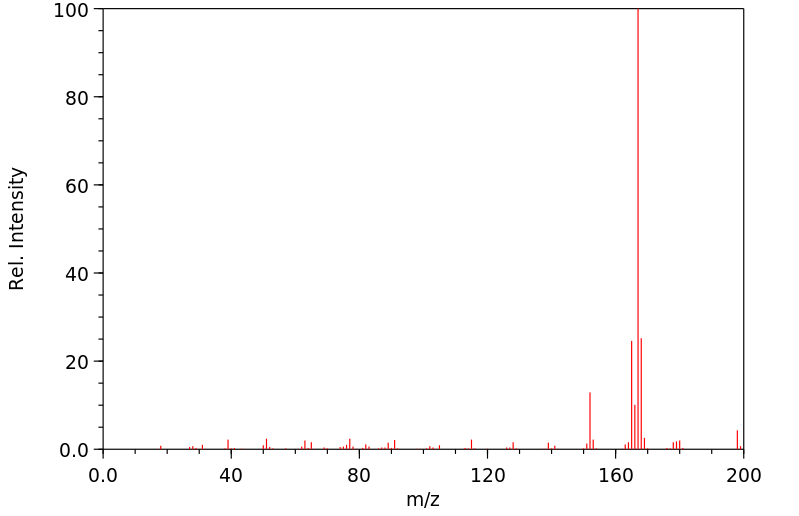
质谱MS
-
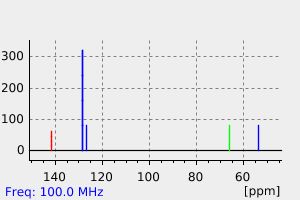
碳谱13CNMR
-
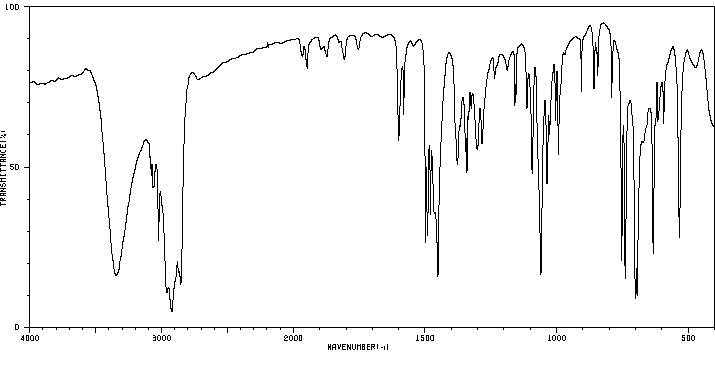
红外IR
-
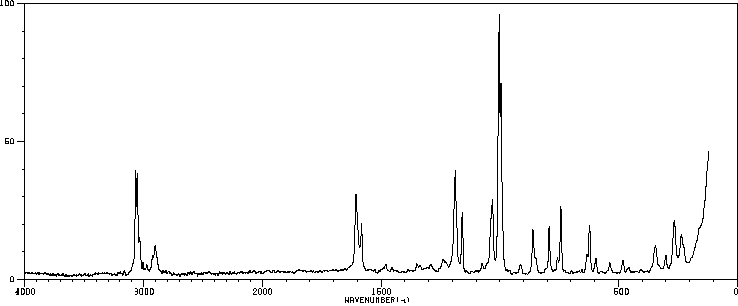
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息