2,3-丁二酮 | 431-03-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-4--2 °C
-
沸点:88 °C(lit.)
-
密度:0.985 g/mL at 20 °C
-
蒸气密度:3 (vs air)
-
闪点:45 °F
-
溶解度:200g/l
-
暴露限值:ACGIH: TWA 0.01 ppm; STEL 0.02 ppmNIOSH: TWA 5 ppb; STEL 25 ppb
-
LogP:-1.340
-
物理描述:Diacetyl appears as a clear colorless liquid with a strong chlorine-like odor. Flash point 80°F. Less dense than water. Vapors heavier than air.
-
颜色/状态:Greenish-yellow liquid
-
气味:Quinone odor; vapors have a chlorine-like odor
-
味道:Taste characteristics at 50 ppm: sweet, buttery, creamy and milky
-
蒸汽密度:3 (NTP, 1992) (Relative to Air)
-
蒸汽压力:56.8 mm Hg at 25 °C
-
亨利常数:1.33e-05 atm-m3/mole
-
大气OH速率常数:2.38e-13 cm3/molecule*sec
-
稳定性/保质期:
-
自燃温度:365 °C
-
分解:When heated to decomposition it emits acrid smoke and fumes.
-
气味阈值:8.6 ppb.
-
折光率:Index of refraction: 1.3951 at 20 °C.
-
保留指数:558;558;558;560;604;586;561;561;572;558;560;613;619;555;550;565;564;613;594;575;575;569.2;578;578;603;562;625;560;563;560;566;569;558;560;558;604;568;563;555;558;563;575;561;570;619;605;565;575.3;582.2
计算性质
-
辛醇/水分配系数(LogP):-1.3
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险等级:3
-
安全说明:S16,S26,S36/37/39,S37/39,S39,S9
-
危险品运输编号:UN 2346 3/PG 2
-
WGK Germany:2
-
海关编码:2914190090
-
危险类别:3
-
危险品标志:F
-
危险类别码:R20/21/22,R36/38,R11
-
RTECS号:EK2625000
-
包装等级:II
-
储存条件:本品应密封保存在阴凉处,存储温度为4°C。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 2,3-丁二酮;二乙酰 |
| 化学品英文名称: | 2,3-Butanedione;Diacetyl |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 431-03-8 |
| 分子式: | C 4 H 6 O 2 |
| 分子量: | 86.09 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:2,3-丁二酮;二乙酰 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | 第3.2类 中闪点易燃液体 |
| 侵入途径: | 吸入 食入 经皮吸收 |
| 健康危害: | 具有刺激性。接触后可引起恶心、头痛和呕吐。 |
| 环境危害: | 对环境有危害,对大气可造成污染。 |
| 燃爆危险: | 本品易燃,有毒,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用流动清水冲洗。 |
| 眼睛接触: | 立即提起眼睑,用流动清水冲洗。 |
| 吸入: | 迅速脱离现场至空气新鲜处。保持呼吸道通畅。必要时进行人工呼吸。就医。 |
| 食入: | 误服者给饮大量温水,催吐,就医。 |
| 第五部分:消防措施 |
| 危险特性: | 其蒸气与空气形成爆炸性混合物,遇明火、高热能引起燃烧爆炸。与氧化剂能发生强烈反应。其蒸气比空气重,能在较低处扩散到相当远的地方,遇火源引着回燃。若遇高热,容器内压增大,有开裂和爆炸的危险。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 泡沫、二氧化碳、干粉、砂土。用水灭火无效。 |
| 消防员的个体防护: | 消防人员须佩戴防毒面具、穿全身消防服 |
| 禁止使用的灭火剂: | |
| 闪点(℃): | 6 |
| 自燃温度(℃): | 无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 疏散泄漏污染区人员至安全区,禁止无关人员进入污染区,切断火源。建议应急处理人员戴自给式呼吸器,穿一般消防防护服。在确保安全情况下堵漏。喷水雾会减少蒸发但不能降低泄漏物在受限制空间内的易燃性。用活性炭或其它惰性材料吸收,收集运至废物处理场所处置。也可以用大量水冲洗,经稀释的洗水放入废水系统。如大量泄漏,利用围堤收容,然后收集、转移、回收或无害处理后废弃。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,注意通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防毒面具(半面罩),戴化学安全防护眼镜,穿防静电工作服,戴橡胶耐油手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、还原剂、碱类接触。灌装时应控制流速,且有接地装置,防止静电积聚。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。库温不宜超过30℃。应与氧化剂、还原剂、碱类、食用化学品分开存放,切忌混储。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 中国MAC:未制定标准苏联MAC:未制定标准美国TWA:未制定标准 TLVWN: 未制定标准 |
| 监测方法: | |
| 工程控制: | 密闭操作,注意通风。 |
| 呼吸系统防护: | 高浓度环境中,佩带防毒面具。 |
| 眼睛防护: | 必要时戴化学安全防护眼镜。 |
| 身体防护: | 穿相应的防护服。 |
| 手防护: | 戴防护手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作后,淋浴更衣。避免长期反复接触。 |
| 第九部分:理化特性 |
| 外观与性状: | 微绿黄色液体,有强烈的气味。 |
| pH: | |
| 熔点(℃): | 无资料 |
| 沸点(℃): | 88 |
| 相对密度(水=1): | 0.99 |
| 相对蒸气密度(空气=1): | 3.00 |
| 饱和蒸气压(kPa): | 无资料 |
| 燃烧热(kJ/mol): | 无资料 |
| 临界温度(℃): | 无资料 |
| 临界压力(MPa): | 无资料 |
| 辛醇/水分配系数的对数值: | 无资料 |
| 闪点(℃): | 6 |
| 引燃温度(℃): | 无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 4 H 6 O 2 |
| 分子量: | 86.09 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 溶于水、乙醇、乙醚。 |
| 主要用途: | 用作食品香料载体。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 稳定 |
| 禁配物: | 强氧化剂、强还原剂、强碱。 |
| 避免接触的条件: | |
| 聚合危害: | 不能出现 |
| 分解产物: | 一氧化碳、二氧化碳。 |
| 第十一部分:毒理学资料 |
| 急性毒性: | LD50:1580mg/kg(大鼠经口) LC50:无资料 |
| 急性中毒: | |
| 慢性中毒: | |
| 亚急性和慢性毒性: | |
| 刺激性: | |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | |
| 生物降解性: | |
| 非生物降解性: | |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | |
| 废弃处置方法: | 处置前应参阅国家和地方有关法规。建议用焚烧法处置。 |
| 废弃注意事项: |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 32081 |
| UN编号: | 2346 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 安瓿瓶外普通木箱;螺纹口玻璃瓶、铁盖压口玻璃瓶、塑料瓶或金属桶(罐)外普通木箱;螺纹口玻璃瓶、塑料瓶或镀锡薄钢板桶(罐)外满底板花格箱、纤维板箱或胶合板箱。 |
| 运输注意事项: | 运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。夏季最好早晚运输。运输时所用的槽(罐)车应有接地链,槽内可设孔隔板以减少震荡产生静电。严禁与氧化剂、还原剂、碱类、食用化学品等混装混运。运输途中应防曝晒、雨淋,防高温。中途停留时应远离火种、热源、高温区。装运该物品的车辆排气管必须配备阻火装置,禁止使用易产生火花的机械设备和工具装卸。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。铁路运输时要禁止溜放。严禁用木船、水泥船散装运输。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定;常用危险化学品的分类及标志 (GB 13690-92)将该物质划为第3.2 类中闪点易燃液体。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | 1.周国泰,化学危险品安全技术全书,化学工业出版社,1997 2.国家环保局有毒化学品管理办公室、北京化工研究院合编,化学品毒性法规环境数据手册,中国环境科学出版社.1992 3.Canadian Centre for Occupational Health and Safety,CHEMINFO Database.1998 4.Canadian Centre for Occupational Health and Safety, RTECS Database, 1989 |
| 填表时间: | 年月日 |
| 填表部门: | |
| 数据审核单位: | |
| 修改说明: | |
| 其他信息: | 1 |
| MSDS修改日期: | 年月日 |
制备方法与用途
微绿黄色液体,具有强烈的气味。
性质散发强烈的奶油香味、发酵香味、乳脂香味和甜香味等。
用途用于配制奶油香精,是生产吡嗪类香料的主要原料;主要用于食品用香精的配制,特别是作为奶油香精的主要成分,也可应用于牛奶、乳酪及其他一些香味中。如在浆果、焦糖、巧克力、咖啡、樱桃、香荚兰豆、蜂蜜、可可、果香、酒香、烟香、朗姆、坚果、杏仁及生姜等食品中。
含量分析按醛和酮测定法(OT-7)中的羟胺法进行。取样500mg,计算时当量因子(e)为21.52。建议使用GT-10-4中非极性柱进行测定。
毒性未规定ADI(FAO/WHO, 1994);GRAS(FEMA;FDA,§184.1278,2000)。
使用限量FEMA(mg/kg):软饮料2.5;冷饮5.9;糖果21;焙烤食品44;布丁类19;胶姆糖35;起酥油11。
食品添加剂最大允许使用量及残留标准 化学性质黄色至黄绿色液体,大量稀释后(1mg/kg时)呈奶油香气。蒸气压高,室温下能迅速挥发。熔点-3~-4℃,沸点87~88℃,闪点13℃。溶于乙醇、乙醚、大多数非挥发性油和丙二醇,也溶于甘油和水,不溶于矿物油。
天然存在于月桂油、香旱芹子油、欧白芷根油、树莓、草莓、奶油、葡萄酒等中。因易挥发,仅存在于精油的初馏分及蒸馏水中。
用途GB 2760-96规定为暂时允许使用的食用香料,主要用于配制奶油、干酪发酵风味和咖啡等型香精。此外,还用于配制各种奶香型食用香精,是奶油、人造奶油、干酪和糖果的增香剂,也用作明胶硬化剂及照相粘结剂。
生产方法在自然界中广泛存在于多种植物的香精油中,如鸢尾油、当归油、月桂油等。工业上通过将甲基乙基酮用亚硝酸处理生成丁酮肟,再用水稀释硫酸分解而得;或采用乙烯基乙炔或甲基乙烯基酮经水合再氧化的方法;实验室可通过二氧化硒氧化丁酮制取,也可采用二酮二肟与亚硝酸钠反应的方法。
生产方法可以从精油中通过游离法制备:精油1份加2份磷酸生成结晶性加成物C4H6O2?2H3PO4,加水后即释出丁二酮。若磷酸加入过量,则形成液体的加成物。
由葡萄糖经特殊发酵获得。
从甲乙酮为起始原料合成。
将丁酮在盐酸存在下用亚硝酸钠氧化后,再用水稀释硫酸分解而得。
类别易燃液体
毒性分级高毒
急性毒性- 大鼠口服LD50: 1580毫克/公斤;
- 小鼠口服LD50: 250毫克/公斤
- 兔子皮肤接触:500毫克/24小时,中度刺激
遇明火、高温或氧化剂易燃;燃烧产生刺激烟雾
储运特性库房通风低温干燥;与氧化剂分开存放
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丁酮 butanone 78-93-3 C4H8O 72.1069 丙酮醛 2-oxopropanal 78-98-8 C3H4O2 72.0636 2-戊酮 2-Pentanone 107-87-9 C5H10O 86.1338 3,4-己二酮 3,4-hexanedione 4437-51-8 C6H10O2 114.144 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丁酮 butanone 78-93-3 C4H8O 72.1069 —— 2,3-dioxosuccinaldehyde 97245-29-9 C4H2O4 114.057 3-甲基-2-丁酮 3-methyl-butan-2-one 563-80-4 C5H10O 86.1338 1-氯-2,3-丁二酮 1-chloro-2,3-dioxo-butane 5559-62-6 C4H5ClO2 120.535
反应信息
-
作为反应物:参考文献:名称:NEW BOOKS摘要:DOI:10.1021/ja01683a042
-
作为产物:描述:acetyl radical 以 gas 为溶剂, 生成 2,3-丁二酮参考文献:名称:乙酰化学在宽温度和压力范围内的反应动力学摘要:分子调制光谱仪已用于研究乙酰基化学中涉及的复杂化学动力学。这涉及两个乙酰基和甲基的基团的直接监测在相同实验中和在各种温度下(263⩽ Ť / K⩽343)和总的气体浓度(0.3⩽[M] / 10 19分子厘米-32.7 2.7)条件。通过对实验数据进行非线性最小二乘法分析和简单的产品研究,对这些测量进行了补充。以此方式确定了四个反应的速率数据和乙酰基在223 nm处的吸收截面。基于Kassel积分的单分子速率理论已应用于压力依赖的自由基的形成和衰变,以提取T = 303和343 K处的速率常数的极限值。DOI:10.1039/f19827802423
-
作为试剂:描述:potassium (4-phenylbutyl)trifluoroborate 、 p-chlorobenzenesulfonyl azide 在 tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate 、 氯化镍二甲氧基乙烷 、 2,3-丁二酮 作用下, 以 乙腈 为溶剂, 反应 24.0h, 以65%的产率得到4-chloro-N-(4-phenylbutyl)benzenesulfonamide参考文献:名称:镍/光氧化还原双催化交叉偶联烷基和酰胺基自由基以构建C(sp 3)–N键摘要:在良性条件下通过直接自由基-自由基交叉偶联来构建C(sp 3)-N键是一种理想但具有挑战性的方法。在此,通过镍/光氧化还原双重催化,以简明,温和且无氧化剂的方式将烷基和and基自由基交叉偶联以建立脂族C–N键。在该协议中,成功地采用了单电子转移策略,分别从磺酰基叠氮化物/叠氮基甲酸酯和烷基三氟硼酸酯生成N-和C-中心的自由基。然而,光催化剂诱导的三重态-三重态能量转移机制可能不适用于该反应。激发的光催化剂(Ru II / * Ru II / Ru III / Ru II的氧化猝灭途径))结合可能的Ni I / Ni II / Ni III / Ni I催化循环,基于协同实验和计算研究,提出了镍/光氧化还原双催化C(sp 3)–N键形成的方法。DOI:10.1021/acscatal.1c00731
文献信息
-
Efficient assembly of oligomannosides using the hydrophobically assisted switching phase method
-
Condensation of Diacetyl with Alkyl Amines: Synthesis and Reactivity of p-Iminobenzoquinones and p-Diiminobenzoquinones作者:Carlos Espinoza-Hicks、Rafael Bautista、Saúl Frias-Puente、Vanessa Pelayo、Eder Martínez-Mora、Francisco Delgado、Joaquín TamarizDOI:10.3390/molecules201119716日期:——Condensation reactions between diacetyl and α-branched primary alkylamines under mild and neutral conditions provided a mixture of 2,5-dimethylbenzoquinone(alkylimines), 2,5-dimethylbenzoquinone(bis-alkyldiimines), and N,N′-dialkyl-2,5-dimethylbenzene-1,4-diamines, which were efficiently separated as pure products by column chromatography. Both 2,5-dimethylbenzoquinone(alkylimines) and 2,5-dimethylbenzoquinone(bis-alkyldiimines) underwent an interchange of the alkylimino group when treated with anilines, followed by reductive aromatization, to provide diarylamines and 1,4-dianilinobenzenes, respectively. Evaluation was also made of the reactivity and selectivity of these compounds in the presence of anilines, thiophenols and alkylhalides.
-
Oxidative Release of Copper from Pharmacologic Copper Bis(thiosemicarbazonato) Compounds作者:John J. Sirois、Lillian Padgitt-Cobb、Marissa A. Gallegos、Joseph S. Beckman、Christopher M. Beaudry、James K. HurstDOI:10.1021/acs.inorgchem.8b00853日期:2018.8.62-Pyridylazoresorcinol complexation was used to demonstrate that Cu(II) release by reaction with peroxynitrite species involved rate-limiting homolysis of the peroxy O–O bond to generate secondary oxidizing radicals (NO2•, •OH, and CO3•–). Because the potentials for CuII(btsc) oxidation and reduction are ligand-dependent, varying by as much as 200 mV, it is clearly advantageous in designing therapeutic methodologies从铜-双-硫代嘧啶铜氮杂铜络合物向细胞内递送治疗性或分析性铜的机制通常涉及内源性还原剂将单电子还原为Cu(I)类似物的机制,从而使金属离子不稳定且与bis--不牢固地配位。硫半脲(bTSc)配体。但是,本文所述的电化学和光谱研究表明,Cu II(bTSc)和Zn II ATSM(bTSc =二乙酰基-双(4-甲基硫代半碳氮杂))复合物的单电子氧化在生理氧化剂范围内发生,导致还存在未被认可的铜释放的氧化途径。H 2 O 2氧化Cu II(bTSc)由髓过氧化物酶或辣根过氧化物酶,HOCl和牛磺酸氯胺(它们主要是由MPO催化反应在活化的中性粒细胞中产生的氯化剂)以及过氧化亚硝酸盐类(ONOOH,ONOOCO 2 –)催化的被证明。与还原不同,氧化反应通过不可逆的配体氧化进行,最终释放出Cu(II)。2-吡啶基偶氮间苯二酚络合物用于证明通过与过氧亚硝酸盐类物质反应释放Cu(II)涉及限速过氧O-
-
一种缩氨脲类化合物的制备方法和在生物医 学方面的应用申请人:华中师范大学公开号:CN105017197B公开(公告)日:2017-07-07本发明提供了一种缩氨脲类化合物的制备方法和在生物医学方面的应用,该化合物为式I所示化合物或其对映异构体、非对映异构体、外消旋体、药学上可接受的盐、结晶水合物或溶剂合物。其中,X为硫或氧;R1和R2分别独立地为氢,含有1~3个碳原子的烷基或N=CHR5,其中R5为任选取代的芳香基或任选取代的烷基;R3和R4分别独立地为氢或含有1~3个碳原子的烷基或选自式II、III、IV、V所示取代基:X1为硫或氧;Y、Y1和Y2分别独立地为氢、含有1~3个碳原子的烷基、卤素、羟基、甲氧基、氨基、磺酸基、硝基、羧基、巯基、甲氨基、乙氨基、二甲氨基或者二乙氨基中的至少一个;Z1为氢、含有1~3个碳原子的烷基、卤素、羟基、氨基、甲氨基、乙氨基、二甲氨基或者二乙氨基;该化合物能够用于铜代谢紊乱引起的相关疾病。
-
Radical-Trapping Antioxidant Activity of Copper and Nickel Bis(Thiosemicarbazone) Complexes Underlies Their Potency as Inhibitors of Ferroptotic Cell Death作者:Omkar Zilka、Jia-Fei Poon、Derek A. PrattDOI:10.1021/jacs.1c08254日期:2021.11.17inhibitor of (phospho)lipid peroxidation. In THF autoxidations, CuATSM reacts with THF-derived peroxyl radicals with kinh = 2.2 × 106 M–1 s–1─roughly 10-fold greater than α-tocopherol (α-TOH), Nature’s best RTA. Mechanistic studies reveal no H/D kinetic isotope effects and a lack of rate-suppressing effects from H-bonding interactions, implying a different mechanism from α-TOH and other canonical RTAs, which在此我们证明了铜 (II)-二乙酰-双 ( N 4 -甲基氨基硫脲) (CuATSM) 是治疗 ALS 和帕金森病的临床候选药物,是一种高效的自由基捕获抗氧化剂 (RTA) 和 (phospho) 的抑制剂脂质过氧化。在 THF 自动氧化中,CuATSM 与 THF 衍生的过氧自由基反应,k inh = 2.2 × 10 6 M –1 s –1─大约是自然界最好的 RTA α-生育酚 (α-TOH) 的 10 倍。机理研究表明,没有 H/D 动力学同位素效应,并且缺乏 H 键相互作用的速率抑制效应,这意味着与 α-TOH 和其他典型 RTA 的机制不同,后者通过 H 原子转移 (HAT) 反应。对于相应的 Ni 2+络合物和 Cu 2+和 Ni 2+的络合物,观察到类似的反应性与其他双(氨基硫脲)配体。计算证实了限速 HAT 不能解释观察到的 RTA 活性的实验发现,而是表明可逆地向双(氨
表征谱图
-
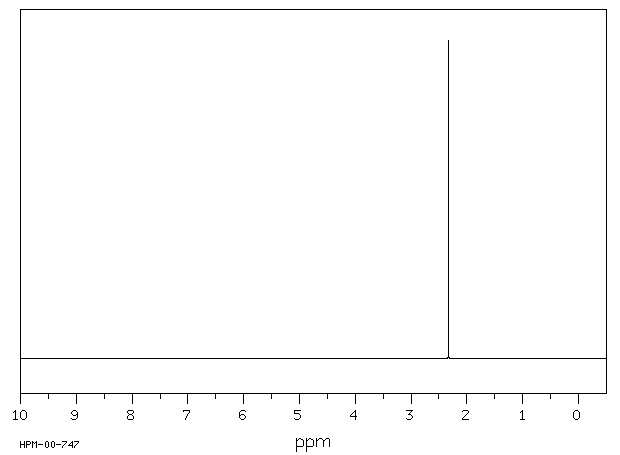
氢谱1HNMR
-
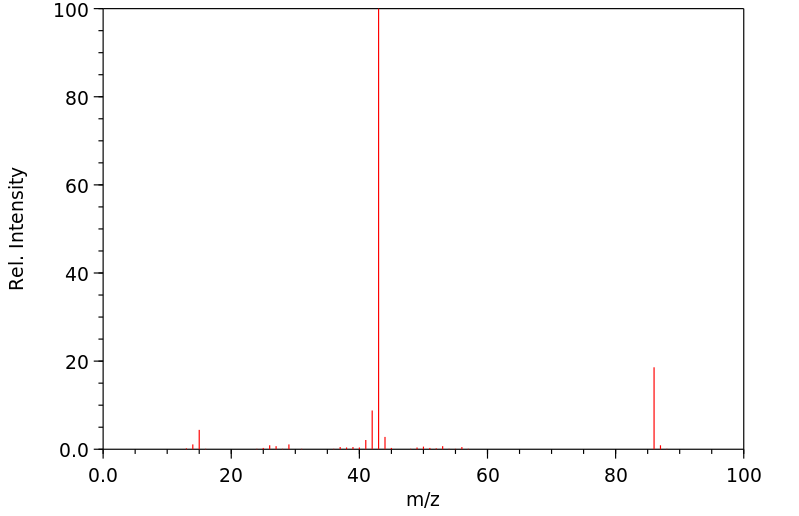
质谱MS
-
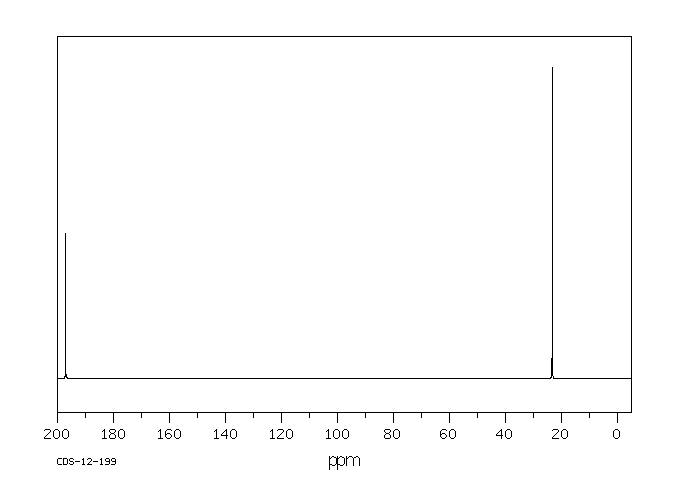
碳谱13CNMR
-
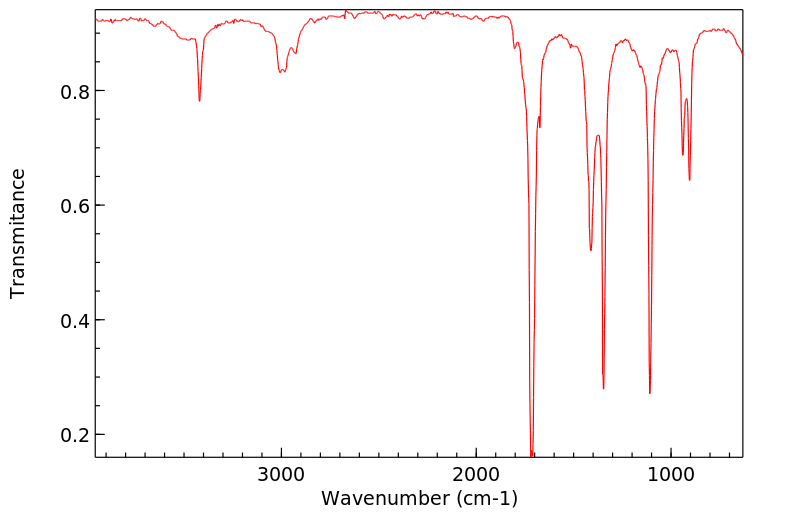
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息