2,6-二甲基苯甲醛 | 1123-56-4
中文名称
2,6-二甲基苯甲醛
中文别名
间二甲苯-2-甲醛
英文名称
2,6-dimethylbenzaldehyde
英文别名
——
CAS
1123-56-4
化学式
C9H10O
mdl
MFCD00128003
分子量
134.178
InChiKey
QOJQBWSZHCKOLL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:27-30 °C (lit.)
-
沸点:230 °C (lit.)
-
密度:1.003±0.06 g/cm3(Predicted)
-
闪点:27 °C
-
保留指数:1640;1640
-
稳定性/保质期:
- 常温常压下稳定。
- 存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2912299000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:常温、避光、存放在通风干燥处。
SDS
2,6-Dimethylbenzaldehyde Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,6-Dimethylbenzaldehyde
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,6-Dimethylbenzaldehyde
Percent: >97.0%(GC)
CAS Number: 1123-56-4
Synonyms: m-Xylene-2-carboxaldehyde
Chemical Formula: C9H10O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
2,6-Dimethylbenzaldehyde
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Lump
Colour: White - Yellow
Odour: No data available
pH: No data available
Melting point/freezing point:27°C (Freezing point)
Boiling point/range: 230°C
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
2,6-Dimethylbenzaldehyde
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,6-Dimethylbenzaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,6-Dimethylbenzaldehyde
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,6-Dimethylbenzaldehyde
Percent: >97.0%(GC)
CAS Number: 1123-56-4
Synonyms: m-Xylene-2-carboxaldehyde
Chemical Formula: C9H10O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
2,6-Dimethylbenzaldehyde
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Lump
Colour: White - Yellow
Odour: No data available
pH: No data available
Melting point/freezing point:27°C (Freezing point)
Boiling point/range: 230°C
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
2,6-Dimethylbenzaldehyde
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,6-Dimethylbenzaldehyde
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基苯甲醛 2-methylphenyl aldehyde 529-20-4 C8H8O 120.151 2,6-二甲基苯甲酸 2,6-dimethyl-benzoic acid 632-46-2 C9H10O2 150.177 2,6-二甲基-苯甲酰氯 2,6-dimethylbenzoyl chloride 21900-37-8 C9H9ClO 168.623 苯甲醛 benzaldehyde 100-52-7 C7H6O 106.124 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三甲苯 1,2,3-trimethylbenzene 526-73-8 C9H12 120.194 2,6-二甲基苯乙酮 2',6'-dimethylacetophenone 2142-76-9 C10H12O 148.205 —— 2-((2,6-dimethylphenyl)ethynyl)benzaldehyde 1422528-98-0 C17H14O 234.298
反应信息
-
作为反应物:参考文献:名称:新型长寿命双杀菌双(dipyrrinato)锌(II)配合物的聚合物封装,可用作光动力疗法光敏剂。摘要:在过去的几年中,使用光动力疗法(PDT)来治疗癌症已受到越来越多的关注。但是,临床上使用的光敏剂(PSs)具有一些局限性,包括水溶性差,肝毒性,光漂白,聚集和从体内清除缓慢,因此,新型PSs的设计引起了人们的极大兴趣。我们提出使用双(dipyrrinato)锌(II)配合物作为有效的PDT PS具有超长的使用寿命。基于重原子效应,这些配合物的系统间交叉将激发态从单重态变为三重态,从而实现了单重态氧的产生。为了克服在水中淬灭作用的局限性并提高水溶性,将铅化合物3包封在聚合物基质中。DOI:10.1002/anie.201907856
-
作为产物:描述:参考文献:名称:PROCESS FOR PREPARING 2,6-DIALKYLPHENYLACETIC ACIDS摘要:该发明涉及一种多阶段工艺,用于通过将2,6-二烷基溴苯与(1)镁,(2)甲酰胺,(3)酸,(4)氢化所得的苯甲醛,(5)激活所得的苄醇,(6)活化苄醇的氰化和(7)水解所得的腈来制备通式(I)的2,6-二烷基苯乙酸。公开号:US20210403406A1
文献信息
-
Conversion of Aryl Aldehydes to Benzyl Iodides and Diarylmethanes by H<sub>3</sub>PO<sub>3</sub>/I<sub>2</sub>作者:Fang Lv、Jing Xiao、Junchun Xiang、Fengzhe Guo、Zi-Long Tang、Li-Biao HanDOI:10.1021/acs.joc.0c02850日期:2021.2.5reductive benzylation reactions with aryl aldehydes. By using a H3PO3/I2 combination, various aromatic aldehydes underwent iodination reactions and Friedel–Crafts type reactions with arenes via benzyl iodide intermediates, readily producing benzyl iodides and diarylmethanes in good yields. Intramolecular cyclization reactions also took place, giving the corresponding cyclic compounds. This new strategy features
-
Antihypertensive substituted imidazole derivatives申请人:Farmos Group, Ltd.公开号:US04544664A1公开(公告)日:1985-10-01The invention provides novel compounds of the formula: ##STR1## wherein the various substituents are defined herein below. Processes for the preparation of these compounds are described, as are novel pharmaceutical compositions comprising at least one of the compounds of their salts. The compounds and their non-toxic salts exhibit valuable pharmacological activity and are useful in the treatment of mammals, especially as antihypertensive agents. Furthermore, some of the compounds have proved to possess antithrombotic and diuretic activity. Antimycotic and antifungal properties have also been found.这项发明提供了以下结构的新化合物:##STR1## 其中各种取代基在此下文中定义。描述了制备这些化合物的方法,以及包含至少一种该化合物或其盐的新型药物组合物。这些化合物及其无毒盐表现出有价值的药理活性,并可用于治疗哺乳动物,特别是作为降压药剂。此外,一些化合物已被证明具有抗血栓和利尿活性。还发现了抗真菌和抗霉菌特性。
-
Palladium‐Catalyzed Reductive Carbonylation of (Hetero) Aryl Halides and Triflates Using Cobalt Carbonyl as CO Source作者:Bhushanarao Dogga、C. S. Ananda Kumar、Jayan T. JosephDOI:10.1002/ejoc.202001328日期:2021.1.15A generalized protocol for the reductive carbonylation of (hetero) aryl halides and triflates under CO gas‐free conditions using Pd/Co2(CO)8 and triethylsilane has been developed. The mild reaction conditions, enhanced safety, and wide substrate scope highlight its importance in routine organic synthesis.
-
Dynamic Kinetic Resolution of Alcohols by Enantioselective Silylation Enabled by Two Orthogonal Transition‐Metal Catalysts作者:Jan Seliger、Martin OestreichDOI:10.1002/anie.202010484日期:2021.1.4A nonenzymatic dynamic kinetic resolution of acyclic and cyclic benzylic alcohols is reported. The approach merges rapid transition‐metal‐catalyzed alcohol racemization and enantioselective Cu‐H‐catalyzed dehydrogenative Si‐O coupling of alcohols and hydrosilanes. The catalytic processes are orthogonal, and the racemization catalyst does not promote any background reactions such as the racemization
-
[EN] CATALYST COMPLEXES WITH CARBENE LIGAND AND METHOD FOR MAKING SAME AND USE IN METATHESIS REACTION<br/>[FR] COMPLEXES DE CATALYSEURS AVEC UN LIGAND CARBÈNE, LEUR PROCÉDÉ DE FABRICATION ET LEUR UTILISATION DANS UNE RÉACTION DE MÉTATHÈSE申请人:GUANG MING INNOVATION COMPANY WUHAN公开号:WO2014108071A1公开(公告)日:2014-07-17This invention relates to catalyst compounds and the synthesis and applications useful in olefin metathesis reactions. The catalyst compounds of the invention are represented by the formula (I): wherein M is a Group 8 metal; X1 and X2 are anionic ligands; L1 and L2 are neutral two electron donor ligands. The present invention also relates to an easy applicable catalyst synthesis and the application in different olefin metathesis processes, e.g. Reaction Injection Molding (RIM), process to make α-olefins from fatty acid ester, e.g. methyl oleate.
表征谱图
-
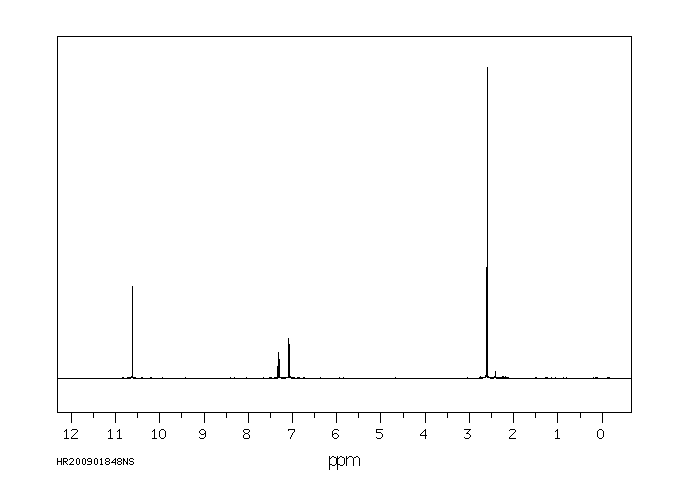
氢谱1HNMR
-
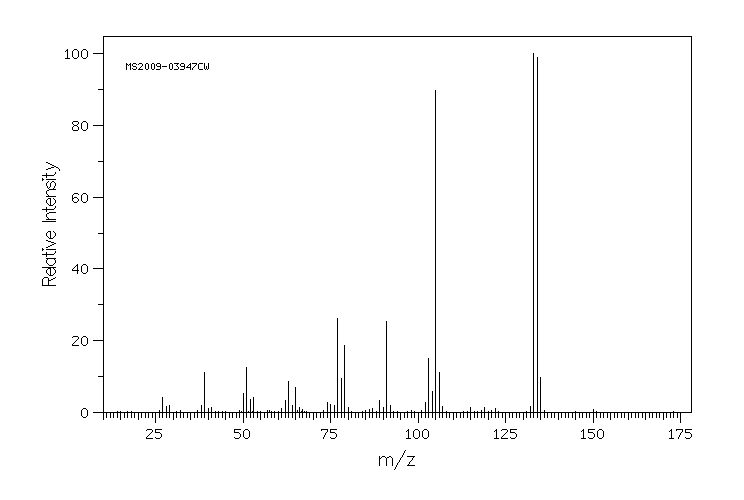
质谱MS
-
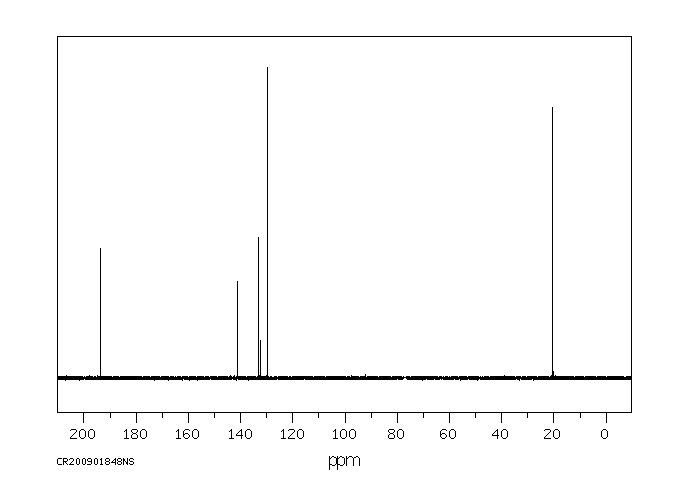
碳谱13CNMR
-
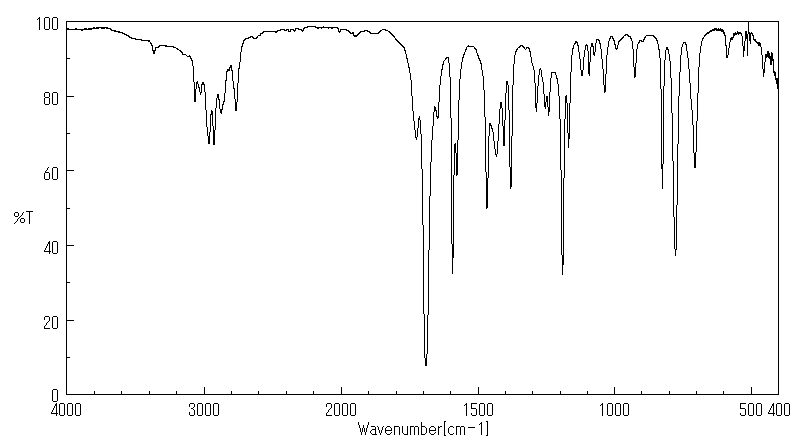
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫