2-(2-苯并噻唑)-5-甲氧基苯酚 | 90481-46-2
物质功能分类
中文名称
2-(2-苯并噻唑)-5-甲氧基苯酚
中文别名
——
英文名称
2-(benzo[d]thiazol-2-yl)-5-methoxyphenol
英文别名
2-(2'-Hydroxy-4'-methoxyphenyl)benzothiazole;2-(2-Benzothiazolyl)-5-methoxyphenol;2-(1,3-benzothiazol-2-yl)-5-methoxyphenol
CAS
90481-46-2
化学式
C14H11NO2S
mdl
MFCD00043777
分子量
257.313
InChiKey
KQUJPMLQKNTPCL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:444.6±55.0 °C(Predicted)
-
密度:1.326±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:18
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.071
-
拓扑面积:70.6
-
氢给体数:1
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(4-甲氧苯基)苯并噻唑 2-(4-methoxy-phenyl)-benzothiazole 6265-92-5 C14H11NOS 241.313
反应信息
-
作为反应物:描述:2-(2-苯并噻唑)-5-甲氧基苯酚 在 甲醇 、 sodium methylate 、 sodium hydride 作用下, 以 四氢呋喃 、 mineral oil 为溶剂, 反应 19.0h, 生成 2-(benzothiazol-2-yl)-5-methoxyphenyl-β-D-galactopyranoside参考文献:名称:2-(Benzothiazol-2-yl)-phenyl-β-d-galactopyranoside derivatives as fluorescent pigment dyeing substrates and their application for the assay of β-d-galactosidase activities摘要:2-(Benzothiazol-2-yl)-phenyl-beta-D-galactopyranoside derivatives were synthesized as novel artificial fluorescent pigment dyeing substrates for beta-D-galactosidase. The substrates, which exhibited non-fluorescence or weak fluorescence in solution phase, were smoothly hydrolyzed by beta-D-galactosidase from Aspergillus oryzae and yielded a water-insoluble strong fluorescent pigment. The difference of fluorescent intensity exhibited a linear relationship with the amount of enzyme. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2013.01.043
-
作为产物:描述:2-(4-甲氧苯基)苯并噻唑 在 2,4,5,6-四(9H-咔唑-9-基)异酞腈 、 氧气 、 palladium diacetate 、 potassium trifluoroacetate 、 三氟乙酸 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 以73%的产率得到2-(2-苯并噻唑)-5-甲氧基苯酚参考文献:名称:通过光氧化还原和钯催化对CH键进行直接加氧。摘要:该报告介绍了通过光催化和Pd催化相结合的CH键的氧合。在这里,我们描述了利用光催化剂将有机钯(II)中间体氧化为高价PdIII或PdIV中间体,从而促进CO键的形成。所证明的方法可以有效地与各种导向基团一起使用,例如肟醚和苯并噻唑。通过合成可用于有机发光二极管和药物的2-(苯并[d]噻唑-2-基)苯酚的几种金属配合物,表明了这种直接CO键形成方法的适用性。DOI:10.1021/acs.joc.9b03197
文献信息
-
[EN] NOVEL USE OF BENZOTHIAZOLE DERIVATIVES<br/>[FR] NOUVELLE UTILISATION DE DERIVES DE BENZOTHIAZOLE申请人:ASTRAZENECA AB公开号:WO2004016600A1公开(公告)日:2004-02-26The use of compounds of formula (I) wherein X, A, B, D, R1 and R2 are as defined in the Specification and pharmaceutically acceptable salts thereof in the manufacture of a medicament for the treatment or prophylaxis of diseases or conditions in which inhibition of kinase Itk activity is beneficial is disclosed. Certain novel compounds of formula (I), together with processes for their preparation, compositions containing them and their use in therapy are also disclosed.公开了在制造用于治疗或预防抑制激酶Itk活性有益的药物时,使用式(I)中X、A、B、D、R1和R2如规范中定义的化合物及其药学上可接受的盐。还公开了式(I)的某些新化合物,以及它们的制备方法、含有它们的组合物和它们在治疗中的用途。
-
Unified synthesis of mono/bis-arylated phenols via Rh<sup>III</sup>-catalyzed dehydrogenative coupling作者:Qian Wu、Ying Chen、Dingyuan Yan、Muyue Zhang、Yi Lu、Wei-Yin Sun、Jing ZhaoDOI:10.1039/c6sc03169b日期:——precise control of the oxidation pathways so that directing groups can be either preserved or cleaved. We found that N-phenoxyacetamides could undergo ortho-arylation reactions with or without an external oxidant, yielding products with different oxidation states, notably the rare bis-arylated phenols. Notably, a unique rhodacycle intermediate was isolated, characterized by X-ray crystallography, and
-
Metallaphotoredox-Mediated C<sub>sp2</sub>–H Hydroxylation of Arenes under Aerobic Conditions作者:Sk. Sheriff Shah、Amrita Paul、Manoranjan Bera、Yarra Venkatesh、N. D. Pradeep SinghDOI:10.1021/acs.orglett.8b01973日期:2018.9.21The direct hydroxylation of 2-arylpyridines and 2-arylbenzothiazoles via the merger of organic photoredox and metal catalysis is reported where 4CzIPN is used as the visible-light photocatalyst and Pd(OAc)2 as the metal catalyst. This method has been employed to synthesize organic molecules exhibiting excited-state intramolecular proton transfer properties for generating tunable luminescence responses
-
Synthesis of bioactive 2-(2-(difluoromethoxy)aryl)benzo[d]thiazole derivatives via base-promoted one-pot process作者:Huiping Zeng、Puying Luo、Manling Luo、Hao Ding、Qiuping DingDOI:10.1016/j.tet.2019.130472日期:2019.8A convenient synthesis of 2-(2-(difluoromethoxy)aryl)benzo[d]thiazoles from 2- (o-hydroxyaryl)benzothiazoles and commercially available ethyl difluoroiodoacetate (ICF2CO2Et) is described. The transformation was amenable to a one-pot, sequential three-component protocol from o-hydroxybenzaldehyde, o-aminothiophenol, and ICF2CO2Et promoted by KOH. Additionally, some of the prepared compounds exhibited
-
Regioselective ortho-hydroxylation of 2-arylbenzothiazole via substrate directed C–H activation作者:Arghya Banerjee、Anupam Bera、Srimanta Guin、Saroj Kumar Rout、Bhisma K. PatelDOI:10.1016/j.tet.2012.12.067日期:2013.3An efficient protocol has been developed for the direct ortho-hydroxylation of 2-arylbenzothiazole using Pd(OAc)2 as the catalyst and either of DIB/AcOH or Oxone®/TFA combinations under air atmosphere. Under both these conditions, the methodology tolerates a diverse array of substituents giving good to excellent yields of corresponding ortho-hydroxylated products. Regioselective hydroxylation is observed一种有效的协议已用于直接被开发邻的羟基化2- arylbenzothiazole使用Pd(OAc)2作为催化剂和任一的DIB / AcOH中或过硫酸氢钾® / TFA在空气气氛下的组合。在这两种条件下,该方法都能耐受各种各样的取代基,从而使相应的邻羟基化产物的收率极佳。在苯环的间位位置具有取代基的苯环的较少空间位阻的位点观察到区域选择性羟基化。
表征谱图
-
氢谱1HNMR
-
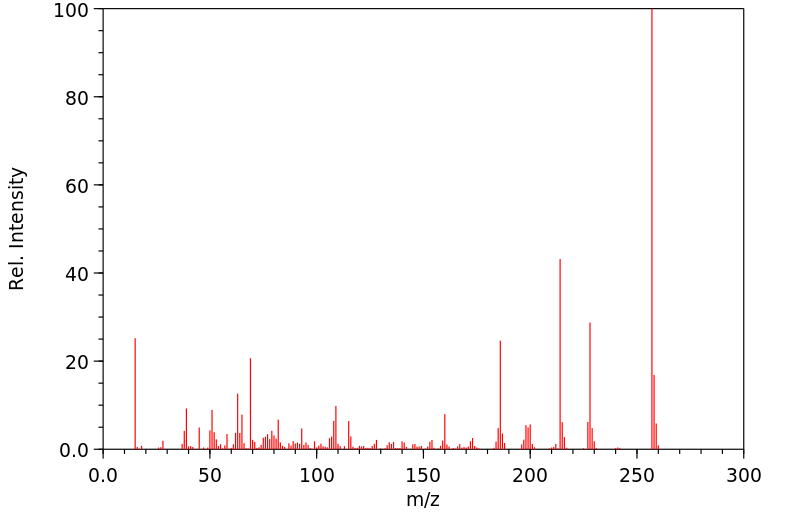
质谱MS
-
碳谱13CNMR
-
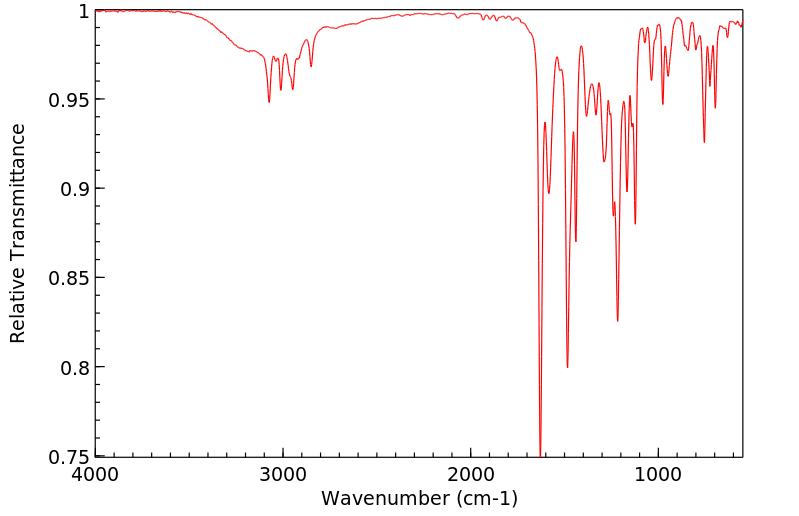
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚