2-氰基-3-甲基丁烯酸乙酯 | 759-58-0
中文名称
2-氰基-3-甲基丁烯酸乙酯
中文别名
2-氰基-3-甲基巴豆酸乙酯
英文名称
ethyl isopropylidenecyanoacetate
英文别名
ethyl 2-cyano-3-methylbut-2-enoate;ethyl 2-cyano-3-methyl-2-butenoate;Ethyl 2-cyano-3-methylcrotonate
CAS
759-58-0
化学式
C8H11NO2
mdl
——
分子量
153.181
InChiKey
PZMDAADKKAXROL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:ca 20℃
-
沸点:98-99 °C/1 mmHg (lit.)
-
密度:1.014 g/mL at 25 °C (lit.)
-
闪点:224 °F
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:11
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:50.1
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S26,S37,S39
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2926909090
-
危险品运输编号:UN 3276
-
包装等级:III
-
危险类别:6.1
-
储存条件:将容器密封后,放入一个紧密封装的储存器中,并储存在阴凉、干燥的地方。
SDS
| Name: | Ethyl 2-cyano-3-methyl-2-butenoate 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 759-58-0 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 759-58-0 | Ethyl 2-cyano-3-methyl-2-butenoate | 98% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 759-58-0: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: colorless to slightly yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 120 - 122 deg C @ 2
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 107 deg C ( 224.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: immiscible with water
Specific Gravity/Density: 1.0140g/cm3
Molecular Formula: C8H11NO2
Molecular Weight: 153.0825
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 759-58-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 2-cyano-3-methyl-2-butenoate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 759-58-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 759-58-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 759-58-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 2-cyanobut-2-enoate 686-33-9 C7H9NO2 139.154 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-ethyl 2-(2-bromo-1-methylethylidene)cyanoacetate 140240-45-5 C8H10BrNO2 232.077 2-氰基-3-甲基丁-2-烯酸 2-cyano-3-methylbut-2-enoic acid 759-21-7 C6H7NO2 125.127 —— 1-Carbethoxy-1-cyan-4-dimethylamino-2-methylbuta-1,3-dien 124571-74-0 C11H16N2O2 208.26
反应信息
-
作为反应物:描述:参考文献:名称:Birch, Journal of the Chemical Society, 1949, p. 2721摘要:DOI:
-
作为产物:参考文献:名称:(±)-(Z)-和(±)-(E)-9-(溴亚甲基)-1,5,5-三甲基螺[5.5] undeca-1,7-dien-3-one和(± )-Majusculone摘要:香米草倍半萜类化合物(Z)-9-(溴亚甲基)-1,5,5-三甲基螺[5.5] undeca-1,7-二烯-3-酮及其15- E表基的新的全合成已于2007年完成。 13个步骤。在我们的序列中,Diels-Alder反应和随后的加合物还原烷基化被用作关键策略,以创建具有合适功能性的A环和季螺中心,以接近B环。此外,还可以通过两步轻松地从高级中间体完全合成去甲香豆素天然产物(±)-大丁烯酮。 变体-倍半萜-大枣-总合成-Diels-Alder反应DOI:10.1055/s-0030-1258412
文献信息
-
β‐Aryl Nitrile Construction<i>via</i>Palladium‐Catalyzed Decarboxylative Benzylation of α‐Cyano Aliphatic Carboxylate Salts作者:Rui Shang、Zheng Huang、Xiao Xiao、Xi Lu、Yao Fu、Lei LiuDOI:10.1002/adsc.201200383日期:2012.9.17The palladium‐catalyzed decarboxylative benzylation of α‐cyano aliphatic carboxylate salts with benzyl electrophiles was discovered. This reaction exhibits good functional group compatibility and proceeds under relatively mild conditions. A diverse range of quaternary, tertiary and secondary β‐aryl nitriles can be conveniently prepared by this method.
-
Flash preparation of carbenoids: A different performance of cyanogen bromide作者:Mohammad Hedayati、Nader PesyanDOI:10.13005/ojc/300477日期:2014.12.31Cyanogen halides are known substances for the cyanating reaction. There are a few evidences for bromination reaction too. On the other hand carbenes are known as very important substances due to their remarkable reactions. Unfortunately carbenes at room temperature are very unstable and there is not a simple method for preparation of them. In most cases the isolation is not possible. We have reported a new reliable and fast preparation method of almost stable carbenoids. The mechanism of the formation has been discussed.
-
Transition metal polyhydrides-catalyzed addition of activated nitriles to aldehydes and ketones via Knoevenagel condensation作者:Yingrui Lin、Xianchao Zhu、Min XiangDOI:10.1016/0022-328x(93)80087-r日期:1993.4Transition metal polyhydrides and dihydrogen complexes catalyze Knoevenagel addition of cyanoacetate to aldehydes and ketones under neutral and mild conditions, the adducts undergo dehydration to give substituted (E)-2-cyano-α,β-unsaturated esters exclusively.
-
Bifunctional Acid-Base Ionic Liquid Organocatalysts with a Controlled Distance Between Acid and Base Sites作者:Mercedes Boronat、Maria J. Climent、Avelino Corma、Sara Iborra、Raquel Montón、Maria J. SabaterDOI:10.1002/chem.200901519日期:2010.1.25Bifunctional acid–base ionic liquid organocatalysts with different distances between the two sites have been synthesised, and their activity for the Knoevenagel condensation has been tested. As has been found to be the case with enzymes, the distance between the acidic and basic sites determines the activity of the bifunctional organocatalyst, and at the optimal distance the reaction rate increases
-
Synthesis of nitrocyclopropanedicarboxylic acid derivatives by addition of α-bromonitroalkanes to methylidene malonic, methylidene cyanoacetic or maleic acid derivatives作者:G. V. Kryshtal、G. M. Zhdankina、A. S. Shashkov、S. G. ZlotinDOI:10.1007/s11172-011-0348-8日期:2011.11A potassium carbonate promoted addition of 2-bromonitroalkanes to methylidenemalonic and methylidenecyanoacetic acid derivatives and N-benzylmaleimide leads to the functionalized nitrocyclopropanes. In the case of less active olefins, it is reasonable to use the phase-transfer catalyst Bu4NPF6 (10 mol.%), whereas in the case of N-benzylmaleimide, to carry out the process in the ionic liquid [bmim]BF4.
表征谱图
-
氢谱1HNMR
-
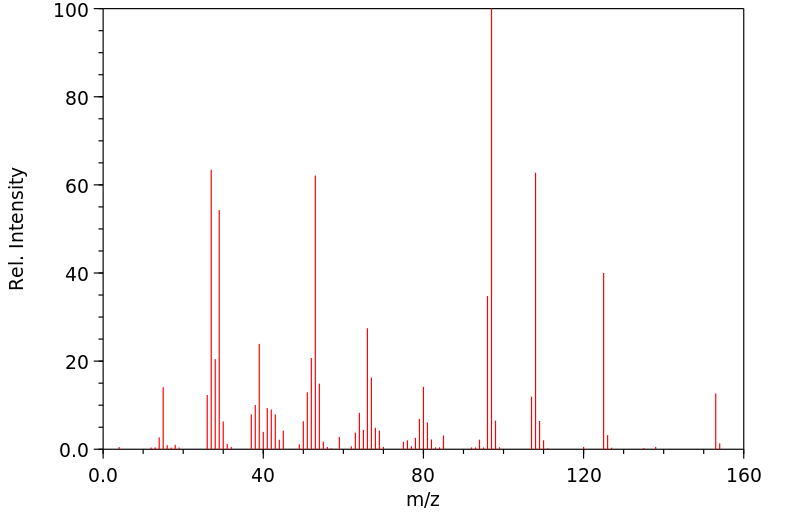
质谱MS
-
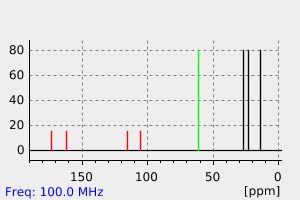
碳谱13CNMR
-
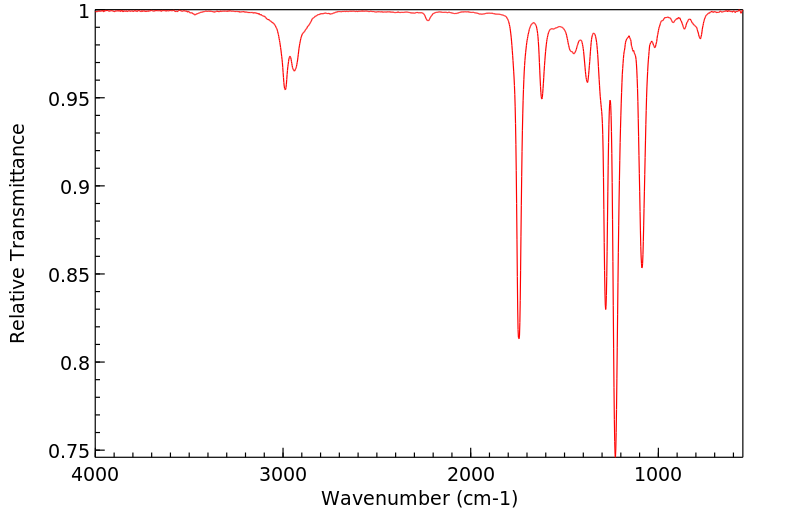
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯