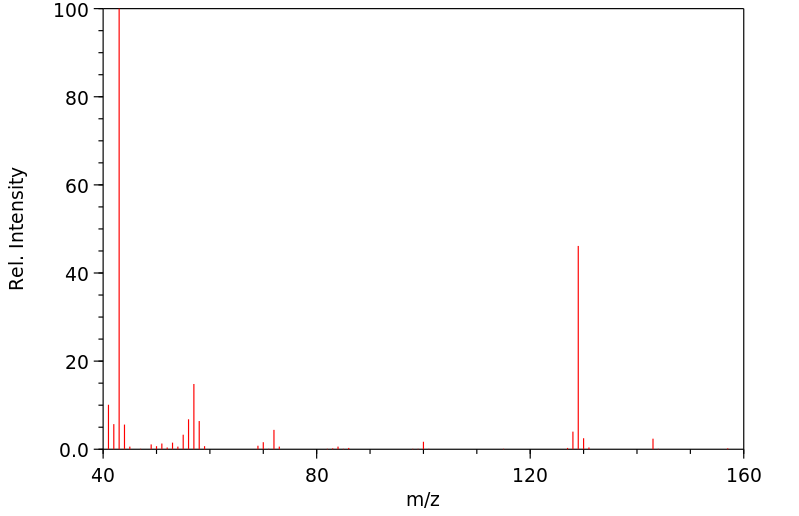
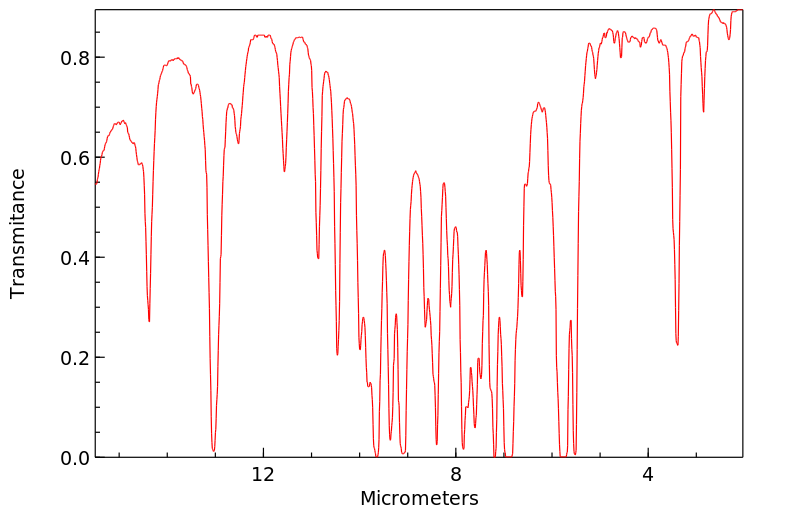
[EN] 2,3,5 TRISUBSTITUTED ARYL AND HETEROARYL AMINO DERIVATIVES, COMPOSITIONS, AND METHODS OF USE<br/>[FR] DÉRIVÉS AMINÉS D'HÉTÉROARYLE ET D'ARYLE 2,3,5 TRISUBSTITUÉS, COMPOSITIONS ET PROCÉDÉS D'UTILISATION ASSOCIÉS
申请人:NEUROTHERAPEUTICS PHARMA INC
公开号:WO2013059648A1
公开(公告)日:2013-04-25
Compounds are disclosed that have a formula represented by the following:(I) wherein Cy, L1 R1, R2a, R2b, R3, R4, n, and L2 are as described herein. These compounds may be prepared as a pharmaceutical composition, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example addictive disorders, Alzheimer's Disease, anxiety disorders, ascites, autism, bipolar disorder, cancer, depression, endothelial corneal dystrophy, edema, epilepsy, glaucoma, Huntington's Disease, inflammatory pain, insomnia, ischemia, migraine, migraine with aura, migraine without aura, neuropathic pain, nociceptive neuralgia, nociceptive pain, ocular diseases, pain, itch, excessive itch, Pruritis, neuropathic pruritis, Parkinson's disease, personality disorders, postherpetic neuralgia, psychosis, schizophrenia, seizure disorders, tinnitus, and withdrawal syndromes.
已披露具有以下表示的化合物的公式:(I),其中Cy、L1、R1、R2a、R2b、R3、R4、n和
L2如本文所述。这些化合物可以制备为药物组合物,并可用于预防和治疗包括人类在内的哺乳动物的各种疾病,例如成瘾障碍、阿尔茨海默病、焦虑障碍、腹
水、自闭症、躁郁症、癌症、抑郁症、内皮角膜营养不良、
水肿、癫痫、青光眼、亨廷顿病、炎症性疼痛、失眠、缺血、偏头痛、伴有先兆的偏头痛、无先兆的偏头痛、神经病性疼痛、伤害性神经痛、伤害性疼痛、眼部疾病、疼痛、瘙痒、过度瘙痒、瘙痒、神经性瘙痒、帕
金森病、人格障碍、带状疱疹后神经痛、精神病、精神分裂症、癫痫障碍、耳鸣和戒断综合症等。