2-甲基-1,3-二氧戊环-2-丙酸乙酯 | 941-43-5
中文名称
2-甲基-1,3-二氧戊环-2-丙酸乙酯
中文别名
3-(2-甲基-1,3-二氧戊环-2-基)丙酸乙酯
英文名称
Ethyl levulinate ethylene ketal
英文别名
ethyl 3-(2-methyl-1,3-dioxolan-2-yl)propanoate;1,3-Dioxolane-2-propanoic acid, 2-methyl-, ethyl ester
CAS
941-43-5
化学式
C9H16O4
mdl
MFCD00154540
分子量
188.224
InChiKey
PLUNGZQWVYCJBJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:106-107 °C(Press: 13 Torr)
-
密度:1.046±0.06 g/cm3(Predicted)
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
LogP:0.860 (est)
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:44.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2932999099
SDS
Version 1.1
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name ETHYL 2-METHYL-1,3-DIOXOLANE-2 - 50 MG
-PROPIONATE
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Not hazardous according to Directive 67/548/EEC.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
ETHYL 941-43-5 213-379-4 None
2-METHYL-1,3-DIOXOLANE-2-PROPIONAT
E
Formula C9H16O4
Molecular Weight 188,2200 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
ALDRICH www.molbase.com
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Exercise appropriate precautions to minimize direct contact with
skin or eyes and prevent inhalation of dust.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid inhalation. Avoid contact
with eyes, skin, and clothing. Avoid prolonged or repeated
exposure.
STORAGE
Conditions of Storage: Keep tightly closed.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Respiratory protection is not required. Where
protection from nuisance levels of dusts are desired, use type N95
(US) or type P1 (EN 143) dust masks.
Hand Protection: Protective gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
ALDRICH www.molbase.com
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 1,114
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SENSITIZATION
Sensitization: Possible sensitizer.
Skin: Prolonged or repeated exposure may cause allergic
reactions in certain sensitive individuals. The preceding data,
or interpretation of data, was determined using Quantitative
Structure Activity Relationship (QSAR) modeling.
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: May be harmful if inhaled. Material may be
irritating to mucous membranes and upper respiratory tract.
Ingestion: May be harmful if swallowed.
12 - Ecological Information
No data available.
13 - Disposal Considerations
ALDRICH www.molbase.com
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
Not hazardous according to Directive 67/548/EEC.
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name ETHYL 2-METHYL-1,3-DIOXOLANE-2 - 50 MG
-PROPIONATE
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Not hazardous according to Directive 67/548/EEC.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
ETHYL 941-43-5 213-379-4 None
2-METHYL-1,3-DIOXOLANE-2-PROPIONAT
E
Formula C9H16O4
Molecular Weight 188,2200 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
ALDRICH www.molbase.com
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Exercise appropriate precautions to minimize direct contact with
skin or eyes and prevent inhalation of dust.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Avoid inhalation. Avoid contact
with eyes, skin, and clothing. Avoid prolonged or repeated
exposure.
STORAGE
Conditions of Storage: Keep tightly closed.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
PERSONAL PROTECTIVE EQUIPMENT
Respiratory Protection: Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US)
or CEN (EU). Respiratory protection is not required. Where
protection from nuisance levels of dusts are desired, use type N95
(US) or type P1 (EN 143) dust masks.
Hand Protection: Protective gloves.
Eye Protection: Chemical safety goggles.
9 - Physical and Chemical Properties
Appearance Physical State: Liquid
Property Value At Temperature or Pressure
pH N/A
BP/BP Range N/A
MP/MP Range N/A
ALDRICH www.molbase.com
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient Log Kow: 1,114
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Stable: Stable.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Carbon monoxide, Carbon dioxide.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
SENSITIZATION
Sensitization: Possible sensitizer.
Skin: Prolonged or repeated exposure may cause allergic
reactions in certain sensitive individuals. The preceding data,
or interpretation of data, was determined using Quantitative
Structure Activity Relationship (QSAR) modeling.
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Skin Absorption: May be harmful if absorbed through the skin.
Eye Contact: May cause eye irritation.
Inhalation: May be harmful if inhaled. Material may be
irritating to mucous membranes and upper respiratory tract.
Ingestion: May be harmful if swallowed.
12 - Ecological Information
No data available.
13 - Disposal Considerations
ALDRICH www.molbase.com
SUBSTANCE DISPOSAL
Contact a licensed professional waste disposal service to dispose
of this material. Dissolve or mix the material with a combustible
solvent and burn in a chemical incinerator equipped with an
afterburner and scrubber. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
Not hazardous according to Directive 67/548/EEC.
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-(2-Methyl-1,3-dioxolan-2-yl)propanoyl chloride 65251-82-3 C7H11ClO3 178.616 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 3-(2-methyl-1,3-dioxolan-2-yl)propanoate 35351-33-8 C8H14O4 174.197 2-甲基-1,3-二氧戊环-2-丙酸 dioxolanne de l'acide tevuliques 4388-57-2 C7H12O4 160.17 —— 2-(2-ethoxycarbonylpropyl)-2-methyl-1,3-dioxolane 97998-33-9 C10H18O4 202.251 3-(2-甲基-1,3-二氧戊环-2-基)丙醛 3-(2-methyl-[1,3]dioxolan-2-yl)-propionaldehyde 24108-29-0 C7H12O3 144.17 4-(2-甲基[1,3]二氧戊环-2-基)丁烷-2-酮 4-(2-methyl-1,3-dioxolan-2-yl)butan-2-one 33528-35-7 C8H14O3 158.197 —— ethyl 2-((2-methyl-1,3-dioxolan-2-yl)methyl)pentanoate 712265-71-9 C12H22O4 230.304 —— 3-(2-methyl-[1,3]dioxolan-2-yl)-propan-1-ol 29021-98-5 C7H14O3 146.186 —— 2,2-ethylenedioxy-5-octanone 104311-67-3 C10H18O3 186.251 —— 4-(2-methyl-1,3-dioxolan-2-yl)butanal 42991-09-3 C8H14O3 158.197 —— 2,2-ethylenedioxy-5-undecanone 55834-23-6 C13H24O3 228.332 —— 2-(4'-acetoxy-3'-oxobutyl)-2-methyldioxolane —— C10H16O5 216.234 —— 2,2-ethylenedioxy-8-ene-5-nonanone 89588-36-3 C11H18O3 198.262 —— 2-(4-diazo-2-oxobutyl)-2-methyldioxolane 188406-86-2 C8H12N2O3 184.195 2-(3-溴丙基)-2-甲基-1,3-二氧戊环 ethylene-cetal de la bromo-5 pentanone-2 24400-75-7 C7H13BrO2 209.083 —— 2,2-(ethylenedioxy)pentan-5-ol-5,5-d2 36651-25-9 C7H14O3 148.17 —— 2,2-ethylenedioxy-8-hydroxyundec-6-en-5-one 110653-37-7 C14H24O4 256.342 - 1
- 2
反应信息
-
作为反应物:描述:2-甲基-1,3-二氧戊环-2-丙酸乙酯 在 吡啶 、 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 为溶剂, 反应 18.0h, 生成 5-Tosyloxy-2,2-ethylenedioxypentane参考文献:名称:Potentiation of cADPR-Induced Ca2+-Release by Methylxanthine Analogues摘要:Caffeine and other methylxanthines are known to induce Ca2+-release from intracellular stores via the ryanodine receptor. In the present work, a range of caffeine analogues, in which methyl groups at the 1 and 7 positions were replaced with alkyl chains containing different functional groups (oxo, hydroxyl, propargyl, ester, and acids), were synthesized. These compounds were then screened for their ability to potentiate Ca2+-release induced by cADPR (an endogenous modulator of ryanodine receptors) in sea urchin egg homogenates. Two of the synthesized methylxanthines, 1,3-dimethyl-7-(7-hydroxyoctyl)xanthine (37) and 3-methyl-7-(7-oxooctyl)-1-propargylxanthine (66), were shown to be more potent than caffeine in potentiating cADPR-induced Ca2+-release, while 1,3-dimethyl-7-(5-ethylcarboxypentyl)xanthine (14) was shown to be more efficacious. The development of new methylxanthine analogues may lead to a better understanding of ryanodine receptor function and could possibly provide novel therapeutic agents.DOI:10.1021/jm980469t
-
作为产物:参考文献:名称:Phosphocholine derivatives having antihypertensive action摘要:磷酸胆碱衍生物和组合物被描述为在温血动物中作为降压剂和治疗高血压的有用药物。公开号:US04640913A1
文献信息
-
NAD(P)<sup>+</sup>–NAD(P)H Models. 65. Photochemical Reductive Desulfonylation of β-Keto Sulfones with Hantzsch Ester作者:Masayuki Fujii、Kaoru Nakamura、Hideyuki Mekata、Shinzaburo Oka、Atsuyoshi OhnoDOI:10.1246/bcsj.61.495日期:1988.2A new procedure for the reductive desulfonylation of β-keto sulfones is described. The reaction proceeds under a photochemical conditions with the Hantzsch ester in pyridine in the presence of ruthenium(II). Various functional groups are unaffected under the reaction conditions. Application of the procedure to the syntheses of certain natural products is also described.
-
Concise Catalytic Asymmetric Total Synthesis of Biologically Active Tropane Alkaloids作者:Armando Córdova、Shuangzheng Lin、Abrehet TseggaiDOI:10.1002/adsc.201100917日期:2012.5.7the total asymmetric synthesis of valuable tropane alkaloids by catalytic stereoselective transformations is disclosed. The power of this approach is exemplified by the concise catalytic enantioselective total syntheses of (+)‐methylecgonine, (−)‐cocaine and (+)‐cocaine as well as the first catalytic asymmetric total syntheses of a cocaine C‐1 derivative and (+)‐ferruginine starting from 5‐oxo‐protected‐α
-
Tricyclic dihydropyrazolone and tricyclic dihydroisoxazolone potassium channel openers申请人:——公开号:US20020007059A1公开(公告)日:2002-01-17Compounds of formula I 1 are useful in treating diseases prevented by or ameliorated with potassium channel openers. Also disclosed are potassium channel opening compositions and a method of opening potassium channels in a mammal.
-
Einige Reaktionen an acetalisierten Ketoestern作者:L. Willimann、H. SchinzDOI:10.1002/hlca.19490320641日期:1949.10.15A. Die nach Salmi, bzw. Kühn leicht zugänglichen cyclischen Acetale bieten noch viele Möglichkeiten nützlicher Anwendung.答:环状缩醛(根据Salmi和Kühn的观点很容易获得)仍然提供了许多有用的应用可能性。
-
<i>N</i>-(Arylmethyl)-2-(or -4)-cyanopyridinium Hexafluoroantimonates as New Useful Catalysts for Acetalization of Carbonyl Compounds作者:Sang-Bong Lee、Sang-Do Lee、Toshikazu Takata、Takeshi EndoDOI:10.1055/s-1991-26467日期:——Carbonyl compounds 1 were converted to the corresponding 1,3-dioxolanes 2 and 1,3-dioxanes 4 with ethylene glycol and 2,2-dimethyl-1,3-propandiol, respectively, in the presence of 1-3 mol% of N-(benzyl, 4-methylbenzyl or 4-methoxybenzyl)-2(or -4)-cyanopyridinium hexafluoroantimonates 3. The catalyst 3d was also effective for the tetrahydropyranylation.
表征谱图
-
氢谱1HNMR
-
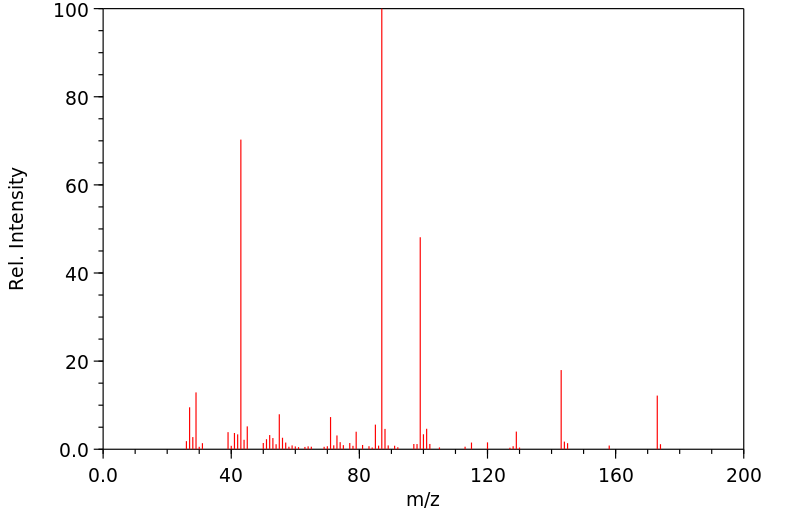
质谱MS
-
碳谱13CNMR
-
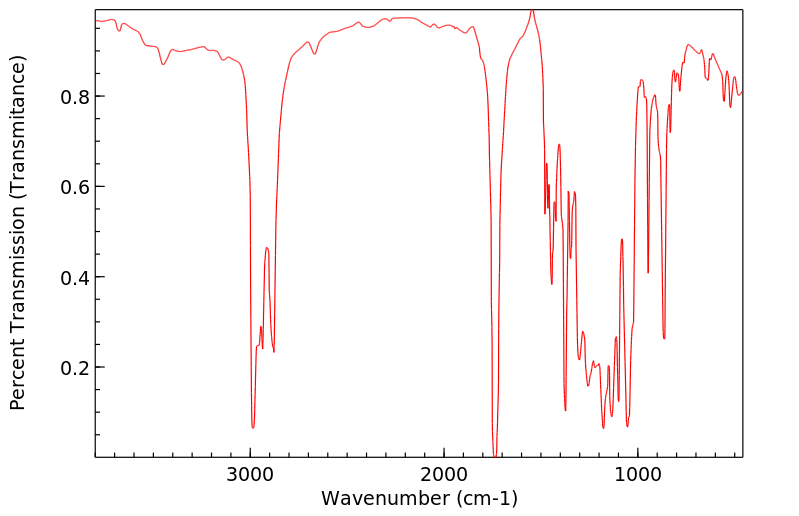
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯