2-甲基-4-辛醇 | 40575-41-5
中文名称
2-甲基-4-辛醇
中文别名
——
英文名称
2-methyloctan-4-ol
英文别名
2-methyl-4-octanol;2-methyl-octan-4-ol
CAS
40575-41-5
化学式
C9H20O
mdl
——
分子量
144.257
InChiKey
BIAVIOIDPRPYJK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:6.15°C (estimate)
-
沸点:192.9°C (estimate)
-
密度:0.8150
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905199090
SDS
反应信息
-
作为反应物:描述:2-甲基-4-辛醇 在 甲酸 、 氯磺酰异氰酸酯 作用下, 以 N,N-二甲基乙酰胺 、 乙腈 为溶剂, 反应 1.0h, 以76%的产率得到(±)-2-methyloctan-4-yl sulfamate参考文献:名称:Ligand-Controlled, Tunable Silver-Catalyzed C–H Amination摘要:The development of readily tunable and regioselective C-H functionalization reactions that operate solely through catalyst control remains a challenge in modern organic synthesis. Herein, we report that simple silver catalysts supported by common nitrogenated ligands can be used to tune a nitrene transfer reaction between two different types of C-H bonds. The results reported herein represent the first example of ligand-controlled and site-selective silver-promoted C-H amination.DOI:10.1021/ja5094309
-
作为产物:参考文献:名称:Solvent-modulated Pd/C-catalyzed deprotection of silyl ethers and chemoselective hydrogenation摘要:Recently we have reported undesirable and frequent deprotection of the TBDMS protective group of a variety of hydroxyl functions occurred under neutral and mild hydrogenation conditions using 10% Pd/C in MeOH. The deprotection of silyl ethers is susceptible to significant solvent effect. TBDMS and TES protecting groups were selectively cleaved in the presence of acid-sensitive functional groups such as TIPS ether, TBDPS ether and dimethyl acetal under hydrogenation condition using 10% Pd/C in MeOH. In contrast, chemoselective hydrogenation of reducible functional groups such as acetylene, olefin and benzyl ether, proceeds in the presence of TBDMS or TES ethers in AcOEt or MeCN. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2004.05.098
文献信息
-
Nonheme Iron-Mediated Amination of C(sp<sup>3</sup>)–H Bonds. Quinquepyridine-Supported Iron-Imide/Nitrene Intermediates by Experimental Studies and DFT Calculations作者:Yungen Liu、Xiangguo Guan、Ella Lai-Ming Wong、Peng Liu、Jie-Sheng Huang、Chi-Ming CheDOI:10.1021/ja3122526日期:2013.5.15(1, qpy = 2,2':6',2″:6″,2''':6''',2''''-quinquepyridine) is a highly active nonheme iron catalyst for intra- and intermolecular amination of C(sp(3))-H bonds. This complex effectively catalyzes the amination of limiting amounts of not only benzylic and allylic C(sp(3))-H bonds of hydrocarbons but also the C(sp(3))-H bonds of cyclic alkanes and cycloalkane/linear alkane moieties in sulfamate esters,7配位络合物[Fe(qpy)(MeCN)2](ClO4)2 (1, qpy = 2,2':6',2″:6″,2''':6''',2' '''-quinquepyridine) 是一种高活性的非血红素铁催化剂,用于 C(sp(3))-H 键的分子内和分子间胺化。该复合物不仅有效地催化有限量的烃的苄基和烯丙基 C(sp(3))-H 键的胺化,而且还催化环烷烃和环烷烃/线性烷烃部分的 C(sp(3))-H 键的胺化。氨基磺酸酯,例如衍生自薄荷烷和甾体胆烷和雄甾烷的那些,使用PhI=NR或“PhI(OAc)2+H2NR”[R=Ts(对甲苯磺酰基),Ns(对硝基苯磺酰基)]作为氮源,胺化产物的分离率高达 93%。亚铁酰亚胺/氮烯中间体 [Fe(qpy)(NR)(X)](n+) (CX, X = NR, 溶剂, 或阴离子)在实验研究的基础上提出,包括 ESI-MS 分析、交叉实验、哈米特图以及与 CH
-
Certain sulfonamide heterobicyclic platelet activating factor antagonists申请人:British Bio-Technology Limited公开号:US05180723A1公开(公告)日:1993-01-19Compounds of general formula I; ##STR1## wherein: A.sup.1 is .dbd.N--, .dbd.CH-- or .dbd.CR.sup.1 --; A.sup.2 is .dbd.N--, .dbd.CH-- or .dbd.CR.sup.2 --; provided that, when one of A.sup.1 and A.sup.2 is a nitrogen atom, the other of A.sup.1 and A.sup.2 is other than a nitrogen atom; wherein the other variables are as defined in the specification and their pharmaceutically and veterinarily acceptable acid addition salts and hydrates are antagonists of platelet activating factor (PAF) and as such are useful in the treatment or amelioration of various diseases or disorders mediated by PAF.通式I的化合物;其中:A.sup.1为.dbd.N--,.dbd.CH--或.dbd.CR.sup.1--; A.sup.2为.dbd.N--,.dbd.CH--或.dbd.CR.sup.2--; 前提是,当A.sup.1和A.sup.2中的一个是氮原子时,另一个不是氮原子;其中其他变量如规范中定义的那样,它们的药用和兽医学可接受的酸盐和水合物是血小板活化因子(PAF)的拮抗剂,因此在治疗或改善由PAF介导的各种疾病或紊乱方面是有用的。
-
A remarkable solvent effect toward the Pd/C-catalyzed cleavage of silyl ethersElectronic supplementary information (ESI) available: characterization data and references and supplementary Tables 4 and 5. See http://www.rsc.org/suppdata/cc/b2/b211313a/
-
[EN] PROCESS<br/>[FR] PROCÉDÉ申请人:PHOSPHAGENICS LTD公开号:WO2018112512A1公开(公告)日:2018-06-28An efficient and commercial phosphorylation process of a complex alcohol, such as secondary and tertiary alcohols, with P4O10 at high temperatures, and a product obtained by the process.
-
Preparation of Single-Enantiomer Biofunctional Molecules with (<i>S</i>)-2-Methoxy-2-(1-naphthyl)propanoic Acid作者:Akio ICHIKAWA、Hiroshi ONODOI:10.1271/bbb.80100日期:2008.9.23(RS)-2-methyl-4-octanol were resolved by using (S)-2-methoxy-2-(1-naphthyl)propanoic acid [(S)-MalphaNP acid]. The specific stereochemistry of each MalphaNP ester was elucidated by 2D NMR analyses, and shielding by the 1-naphthyl group was observed in both the 1H- and 13C-NMR spectra. Solvolysis of the individual (S)-MalphaNP esters gave four single-enantiomer alcohols. The normal-phase HPLC elution order
表征谱图
-
氢谱1HNMR
-
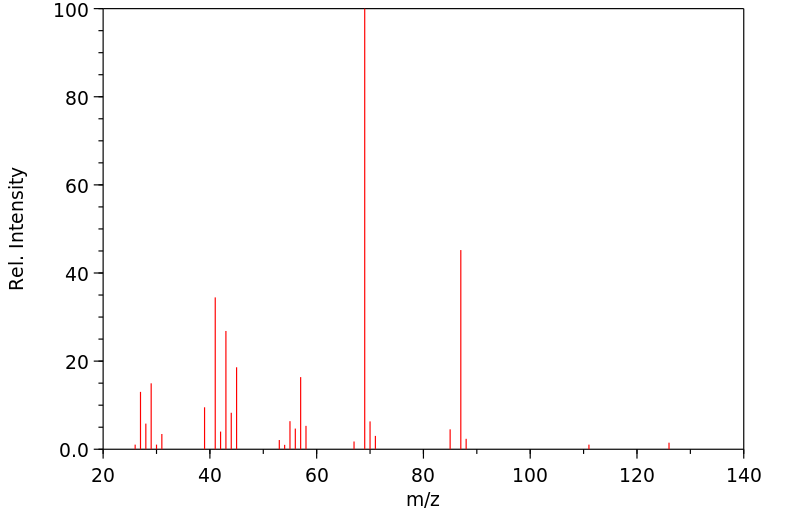
质谱MS
-
碳谱13CNMR
-
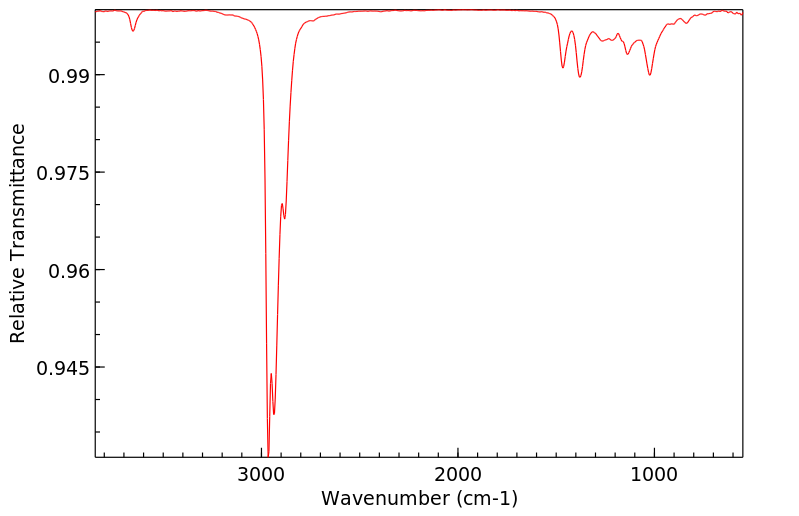
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷