2-碘-3-甲氧基吡啶 | 93560-55-5
中文名称
2-碘-3-甲氧基吡啶
中文别名
2-碘-3-甲氧基吡啶,
英文名称
2-iodo-3-methoxypyridine
英文别名
2-Iod-3-methoxypyridin
CAS
93560-55-5
化学式
C6H6INO
mdl
——
分子量
235.024
InChiKey
NJFRZBAZMPWJKQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:57-61°C
-
沸点:271℃
-
密度:1.825
-
闪点:118℃
-
稳定性/保质期:
避免与光和氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:22.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
危险性防范说明:P233,P260,P261,P264,P270,P271,P280,P301+P312,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P330,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H302,H315,H319,H335
-
储存条件:密封储存于阴凉、干燥的库房,并避免光线照射。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
2-Iodo-3-methoxypyridine
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
2-Iodo-3-methoxypyridine
Ingredient name:
CAS number: 93560-55-5
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H6INO
Molecular weight: 235.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
2-Iodo-3-methoxypyridine
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
2-Iodo-3-methoxypyridine
Ingredient name:
CAS number: 93560-55-5
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H6INO
Molecular weight: 235.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-羟基-2-碘吡啶 2-iodo-3-hydroxypyridine 40263-57-8 C5H4INO 220.997 3-甲氧基吡啶 3-methoxypyridine 7295-76-3 C6H7NO 109.128 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Iod-3-methoxypyridin-1-oxid 115927-86-1 C6H6INO2 251.024 4-羟基-2-碘-3-甲氧基吡啶 2-iodo-3-methoxypyridin-4-ol 405137-17-9 C6H6INO2 251.024
反应信息
-
作为反应物:描述:参考文献:名称:Dehmlow, Eckehard V.; Schulz, Hans-Joachim, Journal of Chemical Research, Miniprint, 1987, # 11, p. 2951 - 2973摘要:DOI:
-
作为产物:描述:3-甲氧基吡啶 在 2,2,6,6-四甲基哌啶 、 正丁基锂 、 二氯(N,N,N',N'-四甲基乙二胺)锌(II) 、 碘 作用下, 以 四氢呋喃 、 正己烷 为溶剂, 反应 0.17h, 以50%的产率得到2-碘-3-甲氧基吡啶参考文献:名称:取代吡啶的原金属化和区域选择性计算的CH酸度关系摘要:室温下,通过使用从ZnCl 2 ·TMEDA(TMEDA = N,N,N ',N'-四甲基乙二胺)和LiTMP(TMP)获得的混合的锂锌混合物,在四氢呋喃中对一系列甲氧基和氟吡啶进行了脱金属化处理。= 2,2,6,6-四甲基哌啶子酮)的比例为1:3,金属化的物质被碘拦截。从4-甲氧基,2-甲氧基,2-甲氧基,2,6-二甲氧基,2-氟和2,6-二氟吡啶观察到在3位的有效官能化,并且从3-甲氧基和2,3-二甲氧基吡啶观察到在4位的高效官能化。有趣的是,注意到3-氟吡啶(在C2和C4处)和2,6-二氟吡啶(在C3和C5处)有干净的双质子化。 根据底物的CH酸(在气相(DFT B3LYP和G3MP2B3含量)和THF溶液中测定)的CH酸性,已经讨论了所获得的区域选择性。在甲氧基吡啶的情况下,还计算了与LiCl和LiTMP的配合物的p K a值。DOI:10.1016/j.tet.2016.03.022
文献信息
-
Ascorbate mediated copper catalyzed reductive cross–coupling of disulfides with aryl iodides作者:Marek Martinek、Michal Korf、Jiri SroglDOI:10.1039/c002725a日期:——The concept of using ascorbic acid as a mediator/ reducing agent in a Cu(I) catalyzed process is introduced and further demonstrated on a crossâcoupling reaction of aryl iodides with disulfides.
-
SPIROPIPERIDINE COMPOUNDS AS ORL-1 RECEPTOR ANTAGONISTS申请人:BENITO COLLADO Ana Belen公开号:US20110118251A1公开(公告)日:2011-05-19An ORL-1 receptor antagonist of the formula: its uses, and methods for its preparation are described.一个公式为ORL-1受体拮抗剂: 其用途和制备方法已被描述。
-
Homoleptic Zincate‐Promoted Room‐Temperature Halogen–Metal Exchange of Bromopyridines作者:Nguyet Trang Thanh Chau、Maxime Meyer、Shinsuke Komagawa、Floris Chevallier、Yves Fort、Masanobu Uchiyama、Florence Mongin、Philippe C. GrosDOI:10.1002/chem.201001664日期:2010.11.2Homoleptic lithium tri‐ and tetraalkyl zincates were reacted with a set of bromopyridines. Efficient and chemoselective bromine–metal exchanges were realized at room temperature with a substoichiometric amount of nBu4ZnLi2⋅TMEDA reagent (1/3 equiv; TMEDA=N,N,N′,N′‐tetramethylethylenediamine). This reactivity contrasted with that of tBu4ZnLi2⋅TMEDA, which was inefficient below one equivalent. DFT calculations
-
NOVEL HETEROCYCLIC DERIVATIVES AS M-GLU5 ANTAGONISTS申请人:Leonardi Amedeo公开号:US20090042841A1公开(公告)日:2009-02-12This invention relates to novel heterocyclic compounds having selective affinity for the mGlu5 subtype of metabotropic receptors, pharmaceutical compositions thereof and uses for such compounds and compositions in the treatment of lower urinary tract disorders, such as neuromuscular dysfunction of the lower urinary tract, and in the treatment of migraine and gastroesophagael reflux disease (GERD).本发明涉及一种新型杂环化合物,其具有选择性亲和力,适用于代谢型受体mGlu5亚型,以及该类化合物的制药组合物及其在治疗下尿路障碍(例如下尿路神经肌肉功能障碍)、偏头痛和胃食管反流病(GERD)方面的用途。
-
Preparation, X-ray Structure, and Reactivity of 2-Iodylpyridines: Recyclable Hypervalent Iodine(V) Reagents作者:Akira Yoshimura、Christopher T. Banek、Mekhman S. Yusubov、Victor N. Nemykin、Viktor V. ZhdankinDOI:10.1021/jo200163m日期:2011.5.20prepared by oxidation of the respective 2-iodopyridines with 3,3-dimethyldioxirane. Structures of 2-iodylpyridine, 2-iodyl-3-isopropoxypyridine, and 2-iodyl-3-propoxypyridine were established by single-crystal X-ray diffraction analysis. 2-Iodyl-3-propoxypyridine has moderate solubility in organic solvents (e.g., 1.1 mg/mL in acetonitrile) and can be used as a recyclable reagent for oxidation of sulfides
表征谱图
-
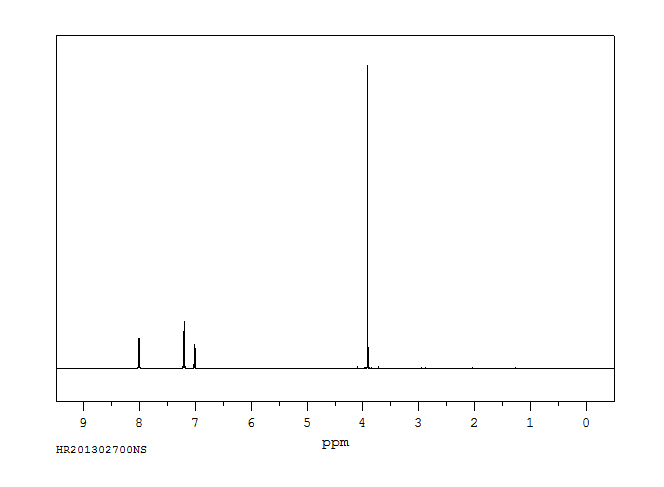
氢谱1HNMR
-
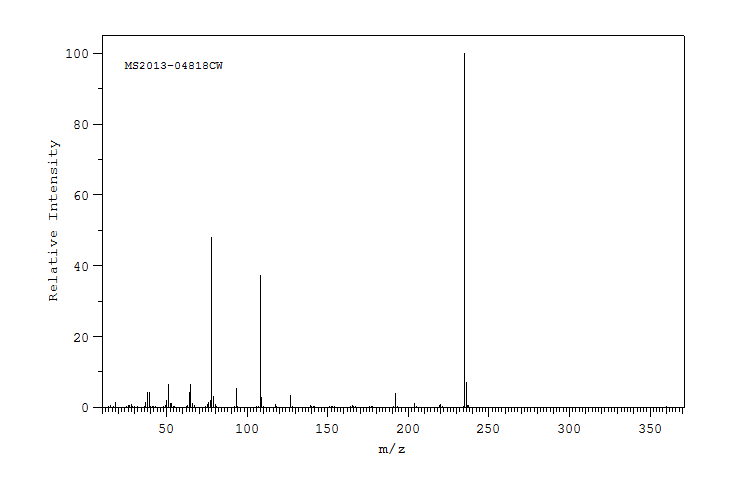
质谱MS
-
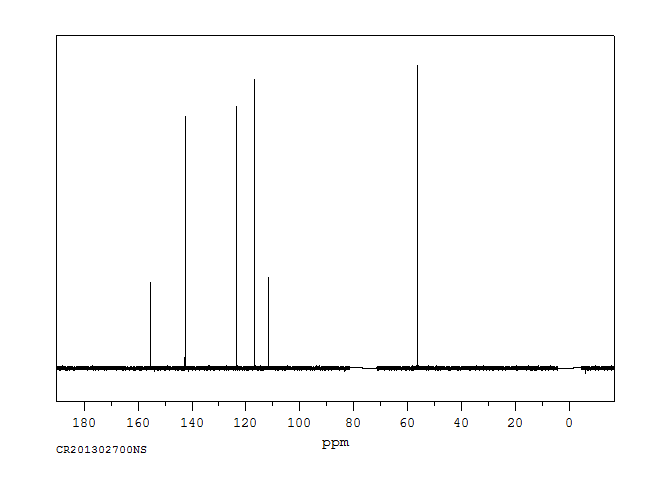
碳谱13CNMR
-
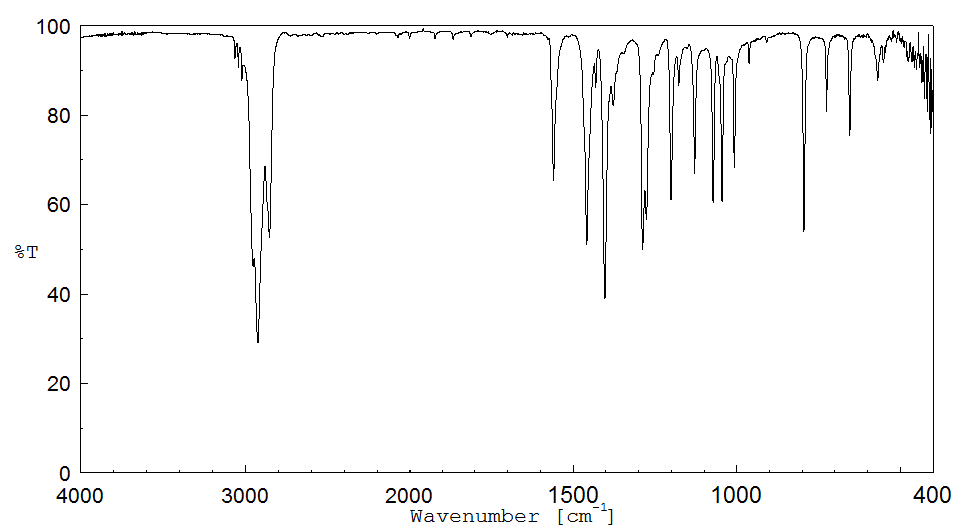
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷