2-苯甲酰-1,3-茚满二酮 | 1785-95-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:108-112 °C
-
沸点:455.4±45.0 °C(Predicted)
-
密度:1.327±0.06 g/cm3(Predicted)
-
稳定性/保质期:
常温常压下稳定,可与强化剂发生反应。
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:19
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.062
-
拓扑面积:51.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S22,S36/37
-
危险类别码:R22
-
海关编码:2914399090
-
RTECS号:NK5105000
-
储存条件:密封贮藏,存放在阴凉干燥处,并远离氧化剂。
SDS
| Name: | 2-Benzoyl-1 3-indanedione 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1785-95-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1785-95-1 | 2-Benzoyl-1,3-indanedione | 98% | unlisted |
Risk Phrases: 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1785-95-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 108 - 112 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density:
Molecular Formula: C16H10O3
Molecular Weight: 250.25
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1785-95-1: NK5105000 LD50/LC50:
CAS# 1785-95-1: Oral, mouse: LD50 = 350 mg/kg.
Carcinogenicity:
2-Benzoyl-1,3-indanedione - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 1785-95-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1785-95-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1785-95-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,3-茚满二酮 1,3-Indandione 606-23-5 C9H6O2 146.145 —— 1-(2-Carboxyphenyl)-3-phenylpropan-1,3-dione 42222-79-7 C16H12O4 268.269 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-[(Z)-{[(Z)-1-methylethyl]imino}(phenyl)methyl]-1H-indene-1,3(2H)-dione —— C19H17NO2 291.349 —— 2-(N-ethoxy-C-phenylcarbonimidoyl)indene-1,3-dione —— C18H15NO3 293.322 —— 2,2-dibromo-indan-1,3-dione 1685-97-8 C9H4Br2O2 303.938
反应信息
-
作为反应物:描述:2-苯甲酰-1,3-茚满二酮 在 甲醇 、 sodium 、 溶剂黄146 作用下, 以 甲醇 为溶剂, 反应 2.0h, 生成 (4Z,6Z)-2,2-diethyl-7-phenyl-4-(p-tolyl)-8H-indeno[2,1-H][1,3,5,6,2]dioxadiazastannonin-8-one参考文献:名称:衍生自2-苯甲酰基-1H-茚-1,3(2H)-二酮和4-取代的苯甲酸酰肼的席夫碱的二有机锡(IV)配合物的合成,表征,体外抗菌,DNA结合活性和QSAR研究摘要:(2021年)。衍生自2-苯甲酰基-1H-茚-1,3(2H)-二酮和4-取代的苯甲酸酰肼的席夫碱的二有机锡(IV)配合物的合成,表征,体外抗菌,DNA结合活性和QSAR研究。磷,硫和硅及相关元素:196,第2号,第133-145页。DOI:10.1080/10426507.2020.1821026
-
作为产物:参考文献:名称:SmCl3催化1,3-二羰基化合物和丙二腈的C-酰化。摘要:已开发出一种可回收,方便且有效的催化体系,用于用酰氯对1,3-二羰基化合物和丙二腈进行C-酰化,在温和条件下产生中等至极好的收率。这是这种反应的第一个催化实例。另外,通过将该方案用作关键步骤,可以轻松地以一锅法高收率合成3,5-二取代-1H-吡唑-4-羧酸酯。DOI:10.1021/ol701961z
文献信息
-
Palladium-Catalyzed Carbonylative Annulation Reactions Using Aryl Formate as a CO Source: Synthesis of 2-Substituted Indene-1,3(2<i>H</i>)-dione Derivatives作者:Ying Zhang、Jing-Lei Chen、Zhen-Bang Chen、Yong-Ming Zhu、Shun-Jun JiDOI:10.1021/acs.joc.5b01758日期:2015.11.6An efficient synthesis of 2-substituted indene-1,3(2H)-diones from stable and readily available 1-(2-halophenyl)-1,3-diones by employing phenyl formate as a CO source has been developed. The reaction occurred via palladium-catalyzed intramolecular carbonylative annulation using K3PO4 as a base and DMSO as a solvent at 95 °C. In this protocol, the reaction showed a broad substrate scope with good to
-
Reactions of 2-Acyl-1,3-indandiones with Nitrogen Nucleophiles作者:Pavel Hrnčiar、Eva ŠvanygováDOI:10.1135/cccc19942734日期:——
Reactions of 2-acyl-1,3-indandiones with nitrogen nucleophiles were studied rarely. The question, if they react with carbonyl carbon of acyl group or indandione skeleton, has not been answered unambiguously. To make clear the question which carbonyl carbon of 2-acyl-1,3-indandiones enters the reaction with nitrogen nucleophiles we carried out the reactions with 2-acetyl- (
Ia ), 2-propionyl- (Ib ), 2-pivaloyl- (Ic ), and 2-benzoyl-1,3-indandione (Id ). We used different 2-acyl-1,3-indandiones with the aim to find out if the character of acyl group affects the course of reaction. We used ethoxyamine, primary amines, phenylhydrazine, hydrazine and methylhydrazine as nucleophile reactants. The reactions were carried out in methanol at reflux at 10% excess of nitrogen base. The reactions with phenylhydrazine, hydrazine and methylhydrazine were performed with twofold excess of nitrogen base. The separation of reaction products was carried out by chromatography on silica gel. We found that 2-acyl-1,3-indandionesI react with ethoxyamine both at the acylcarbonyl carbon to produce 2-(1-ethoxyiminoalkyl)-1,3-indandionesII and the carbonyl carbon of indandione skeleton to give rise 3-(ethoxyimino)-2-acyl-1-indanonesIII . In all cases, the carbonyl carbon of acyl group was preferred (the observed ratio of productsII toIII was 6 - 8 : 1). From the reaction of 2-acyl-1,3-indandiones with primary amines only the productsIV of reaction with the acylcarbonyl carbon were isolated. The hydrazines used reacted with 2-acyl-1,3-indandiones also at carbonyl carbon of acyl group in the first step to produce hydrazones. However, the products isolated in most cases were formed by the attack of hydrazone nitrogen at carbonyl carbon of indandione skeleton giving rise to derivatives of indeno[2,3-d ]pyrazole-4-oneV . It is interesting that 2-acetyl-1,3-indandione and 2-propionyl-1,3-indandione, reacting with phenylhydrazine and hydrazine, yielded only corresponding hydrazonesVI .2-酰基-1,3-茚酮与氮亲核试剂的反应很少被研究。问题是,它们是与酰基羰基还是茚酮骨架上的羰基碳反应,尚未明确回答。为了澄清2-酰基-1,3-茚酮的哪个酰基碳与氮亲核试剂进入反应,我们进行了与2-乙酰-(Ia)、2-丙酰-(Ib)、2-戊酰-(Ic)和2-苯甲酰-1,3-茚酮(Id)的反应。我们使用不同的2-酰基-1,3-茚酮,旨在找出酰基群的性质是否影响反应的过程。我们使用乙氧胺、一级胺、苯基肼、肼和甲基肼作为亲核试剂。反应在甲醇中以10%的氮碱过量进行。苯基肼、肼和甲基肼的反应是在氮碱的两倍过量下进行的。反应产物的分离是通过硅胶色谱法进行的。我们发现,2-酰基-1,3-茚酮I与乙氧胺反应,既在酰基羰基碳上产生2-(1-乙氧亚胺烷基)-1,3-茚酮II,又在茚酮骨架的羰基碳上产生3-(乙氧亚胺)-2-酰基-1-茚酮III。在所有情况下,酰基羰基碳被优先选择(观察到的产物II与III的比例为6-8:1)。从2-酰基-1,3-茚酮与一级胺的反应中,只分离出与酰基羰基碳反应的产物IV。使用的肼也在首步与2-酰基-1,3-茚酮的酰基羰基碳反应,产生肼酮。然而,在大多数情况下,分离出的产物是由肼酮氮原子攻击茚酮骨架的羰基碳形成的,形成吲哚[2,3-d]吡唑-4-酮衍生物V。有趣的是,2-乙酰-1,3-茚酮和2-丙酰-1,3-茚酮与苯基肼和肼反应,只产生相应的肼酮VI。 -
Unraveling Structure−Reactivity Relationships in S<sub>N</sub>V Reactions: Kinetics of the Reactions of Methoxybenzylidenemalononitrile, 2-(Methylthiobenzylidene)-1,3-indandione, 2-(Benzylthiobenzylidene)-1,3-indandione, and Methyl β-Methylthio-α-nitrocinnamate with OH<sup>-</sup> and Thiolate Ions in Aqueous DMSO作者:Claude F. Bernasconi、Rodney J. Ketner、Mark L. Ragains、Xin Chen、Zvi RappoportDOI:10.1021/ja003536t日期:2001.3.1The kinetics of the title reactions were determined in 50% DMSO-50% water (v/v) at 20 degrees C; n-BuS-, HOCH2CH2S-, and MeO2CCH2S- were used as thiolate ions. The reactions with the thiolate ions gave rise to two separate kinetic processes. The first refers to rapid, reversible attachment of RS- to the substrate leading to a tetrahedral intermediate (k1RS), k(-1)RS, the second to the conversion of
-
Fused Tetrazolo[1,5-<i>a</i>]pyrimidines and 2-Azidopyrimidines from 2-Acyl-1,3-indandiones and 5-Aminotetrazole作者:Thomas Russ、Jan W Bats、Walter RiedDOI:10.1055/s-1990-26994日期:——Cyclocondensations of 2-acyl-1,3-indandiones 1a-e and 5-amino-tetrazole monohydrate (2) in ethanol in the presence of catalytic amounts of acetic acid afford indeno[1,2-d]tetrazolo[1,5-a]pyrimidin-6-ones 3a-c or 2-azidoindeno[1,2-d]pyrimidin-5-ones 4a,b in a single step depending on the nature of the acyl substituent R. The structure of 3c was confirmed by X-ray analysis.
-
Photochemical synthesis of isomeric (E/Z)-3-alkylidene-3H-isobenzofuranones作者:Satbir Mor、Som N. Dhawan、Mona Kapoor、Devinder KumarDOI:10.1016/j.tet.2006.11.021日期:2007.1The synthesis of isomeric (E/Z)-3-alkylidene-3H-isobenzofuranones by photoisomerization of 2-aroyl-2-methyl/benzylindan-1,3-diones in high yields is described.
表征谱图
-
氢谱1HNMR
-
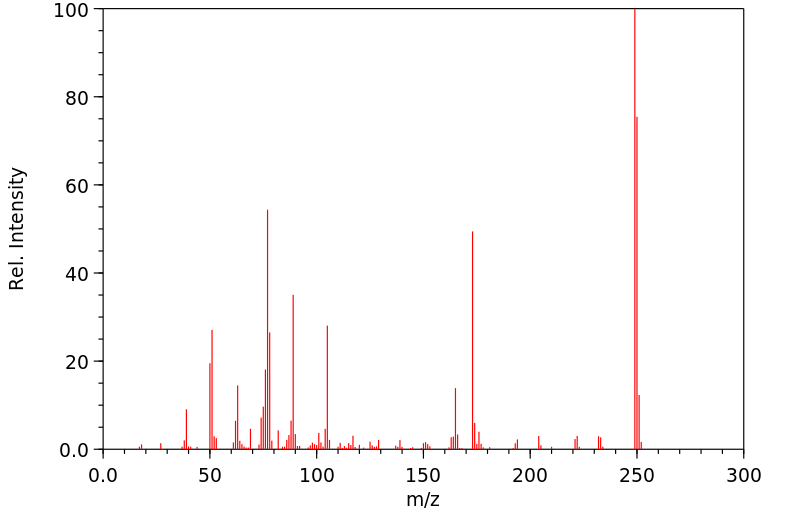
质谱MS
-
碳谱13CNMR
-
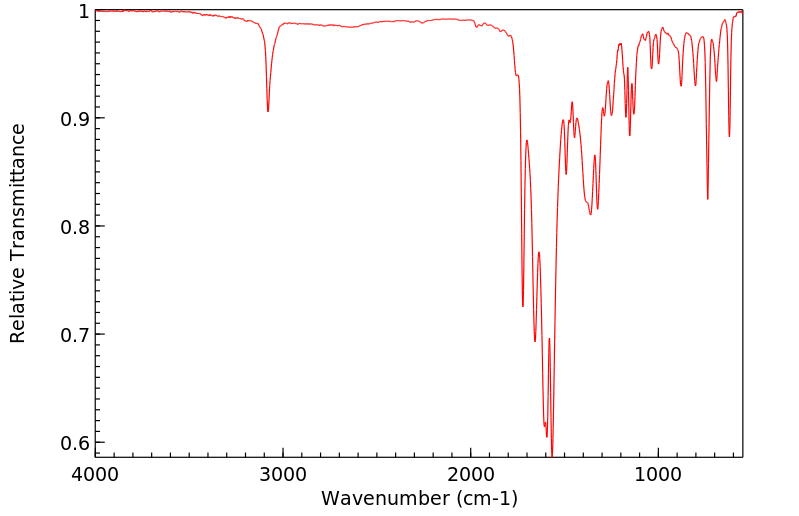
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息