3,3-二苯基-3H-萘并[2,1-b]吡喃 | 4222-20-2
中文名称
3,3-二苯基-3H-萘并[2,1-b]吡喃
中文别名
3,3,3-二苯基-3H-萘并[2,1-B]吡喃
英文名称
3,3-diphenyl-3H-naphtho[2,1-b]pyran
英文别名
3,3-diphenyl-3H-benzo[f]chromene;3,3-diphenyl-naphtho[2,1-b]pyran;3,3-diphenylbenzo[f]chromene
CAS
4222-20-2
化学式
C25H18O
mdl
MFCD00369541
分子量
334.417
InChiKey
UBNNJVRNPJVYBU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:160°C
-
沸点:511.1±50.0 °C(Predicted)
-
密度:1.187±0.06 g/cm3(Predicted)
-
稳定性/保质期:
常温常压下稳定,是一种无色液体,并且可以溶解于水。
计算性质
-
辛醇/水分配系数(LogP):6.7
-
重原子数:26
-
可旋转键数:2
-
环数:5.0
-
sp3杂化的碳原子比例:0.04
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别码:R36/37/38
-
海关编码:2902909090
-
安全说明:S26,S36/37/39
-
危险性防范说明:P501,P273,P391,P270,P264,P301+P312+P330
-
危险性描述:H302,H411
SDS
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块 1. 化学品
产品名称: 3,3-Diphenyl-3H-naphtho[2,1-b]pyran
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,3-二苯基-3H-萘并[2,1-b]吡喃
百分比: >98.0%(LC)
CAS编码: 4222-20-2
分子式: C25H18O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
160°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 3,3-Diphenyl-3H-naphtho[2,1-b]pyran
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 3,3-二苯基-3H-萘并[2,1-b]吡喃
百分比: >98.0%(LC)
CAS编码: 4222-20-2
分子式: C25H18O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
160°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
3,3-二苯基-3H-萘并[2,1-b]吡喃 修改号码:5
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:3,3-二苯基-3H-萘并[2,1-b]吡喃 以 N,N-二甲基甲酰胺 为溶剂, 反应 12.0h, 以66%的产率得到2-benzhydryl-naphtho[2,1-b]furan参考文献:名称:3H-萘并[2,1-b]吡喃的光化学反应性:光致异构化的一个例子摘要:3.3-二苯基-3H-萘并[2.1-b]吡喃的紫外线照射通常诱导吡喃环裂解和吡喃和开环形式之间的热力学平衡:我们观察母体化合物和两个8-取代的衍生物,再循环导致起始吡喃或呋喃化合物。对于 8-ethynvl 3.3-diphenyl-3H-naphtho[2.1-b]pvran。讨论了固体或液体基质的影响。引言 光致变色化合物的结构和光学特性受光能控制,引起了广泛的关注 [1-8]。这些有机系统可能用作逻辑器件和光调制器中的光控光开关。多年来,我们的实验室一直在研究包括 3H-萘并 [2.1-b]pvranic 系统在内的铬化合物,因为它们的性质看起来很有趣,主要用于光敏光学玻璃[9]。λ 在合适的紫外线照射下,色烯的光致变色反应涉及吡喃环的 C(sp )-0 键断裂和扩展的 π 共轭,导致开闭形式平衡(方案 1)。DOI:10.1515/hc.2002.8.1.27
-
作为产物:参考文献:名称:A strategy for the design of photochromic naphthopyrans with large optical density at photosteady state and fast fading speed at ambient temperature in the dark摘要:研究人员描述了一种光致变色萘系化合物,在这种化合物中,有色形式的光密度和褪色速度都显著提高。3 芳基-3-(α-萘)-3H-萘并[2,1-b]吡喃具有热稳定性,在环境温度下可获得较大的有色光密度。通过在α-萘基团上连接烷氧基,有色形式的褪色速度大大加快,而且褪色速度随着烷氧基链长度的增加而加快。该体系的通用性既有利于设计光密度大、在常温下褪色速度快的萘分子,也有利于其应用。DOI:10.1039/c000610f
文献信息
-
Access to Electron-Deficient 2,2-Disubstituted Chromanes: A Highly Regioselective One-Pot Synthesis via an Inverse-Electron-Demand [4 + 2] Cycloaddition of <i>ortho</i>-Quinone Methides作者:Kenta Tanaka、Mami Kishimoto、Yosuke Asada、Yuta Tanaka、Yujiro Hoshino、Kiyoshi HondaDOI:10.1021/acs.joc.9b02036日期:2019.11.1We report the one-pot synthesis of 2,2-disubstituted chromanes with electron-withdrawing substituents. This reaction provides a simple yet efficient route to a wide range of electron-deficient chromanes in high yield and excellent regioselectivity. The reaction of salicylaldehyde with 1,1-disubstituted ethylenes smoothly furnishes these electron-deficient chromanes, which can be further transformed
-
Synthesis and photochromic properties of substituted naphthopyran compounds作者:Zhen Wang、Qinghua Meng、Zhihui Zhang、Dingliang Fu、Wanbin ZhangDOI:10.1016/j.tet.2011.01.078日期:2011.3of 3,3-diaryl-3H-naphtho[2,1-b]pyran compounds with functional substituents at the 5- and 6-positions of the naphthopyran skeleton and the para positions at the 3-aryl moieties were prepared through condensation reactions between 2-naphthol derivatives and 1,1-diarylprop-2-yn-1-ol derivatives. The chemical structures of the compounds were confirmed by NMR and MS. The crystal structure of 3,3-diphen
-
Temperature-controlled divergent synthesis of 4-alkoxy- or 4-alkenyl-chromanes via inverse electron-demand cycloaddition with in situ generated ortho-quinone methides作者:Kenta Tanaka、Mami Kishimoto、Yujiro Hoshino、Kiyoshi HondaDOI:10.1016/j.tetlet.2018.03.090日期:2018.5The temperature-controlled divergent synthesis of 4-alkoxy- or 4-alkenyl-chromanes via inverse electron-demand cycloaddition with in situ generated ortho-quinone methides under identical reaction conditions except for thermal condition has been developed. At room temperature, the reaction generated 4-methoxychromanes, whereas the reaction performed at room temperature to 100 °C gave 4-alkenylchromanes
-
Photochromic Behaviour of Bis[4-(N,N-dimethylamino)phenyl]-substituted 3 H-Naphtho[2,1-b]pyran and 2 H-1-Benzopyran作者:Guénaëlle Harié、André Samat、Robert Guglielmetti、Denis De Keukeleire*、Wim Saeyens、Inge Van ParysDOI:10.1016/s0040-4039(97)00548-0日期:1997.4The photochromic behaviour of 3,3-bis[4-(N,N-dimethylamino)phenyl]-3 H-naphtho[2,1-b]pyran (2) and 2,2-bis[4-(N,N-dimethylamino)phenyl]-2 H-1-benzopyran (3) has been studied by flash photolysis and compared to 3,3-diphenyl-3 H-naphtho[2,1-b]pyran (1) in order to delineate effects due to the N,N-dimethylamino groups. © 1997 Elsevier Science Ltd.
-
Facile One-Pot Synthesis of Photochromic Pyrans作者:Weili Zhao、Erick M. CarreiraDOI:10.1021/ol035599x日期:2003.10.1[reaction: see text]. Photochromic pyrans, including [3H]naphtho[2,1-b]pyrans, [2H]naphtho[1,2-b]pyrans, indeno-fused naphtho[1,2-b]pyrans, and heteroannulated pyrans, were synthesized in excellent yields through a facile one-pot procedure by reaction of propargyl alcohol and naphthol or phenol derivatives in the presence of 5 mol % PPTS and 2 equiv of (MeO)3CH. Symmetrical and nonsymmetrical bispyrans
表征谱图
-
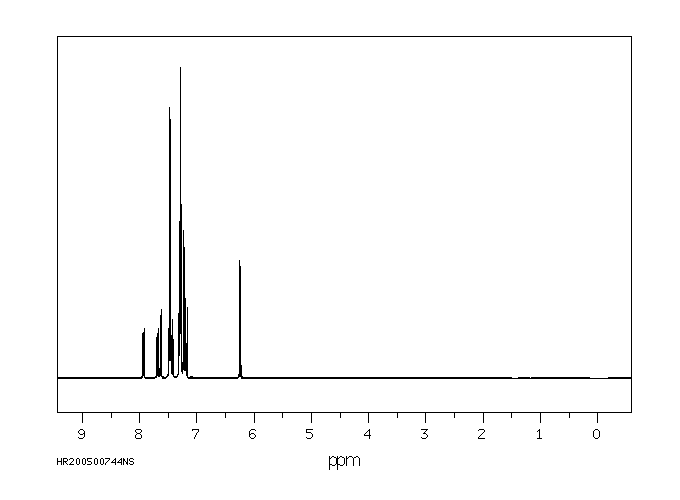
氢谱1HNMR
-
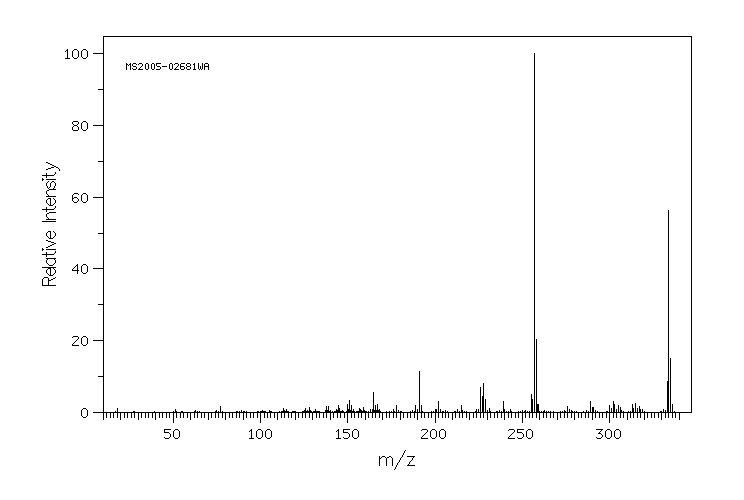
质谱MS
-
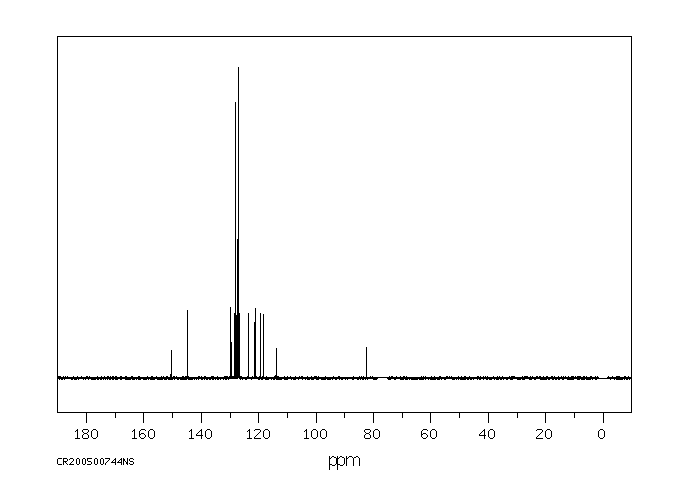
碳谱13CNMR
-
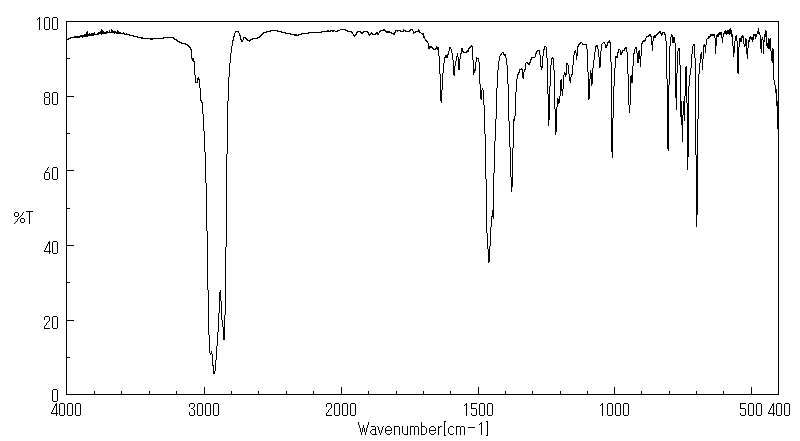
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷