3,5-二叔丁基-4-羟基苯甲腈 | 1988-88-1
中文名称
3,5-二叔丁基-4-羟基苯甲腈
中文别名
3,5-二-叔-丁基-4-羟基苯甲腈
英文名称
2,6-di-tert-butyl-4-cyanophenol
英文别名
3,5-Di-tert-butyl-4-hydroxybenzonitrile;3,5-ditert-butyl-4-hydroxybenzonitrile
CAS
1988-88-1
化学式
C15H21NO
mdl
MFCD00156137
分子量
231.338
InChiKey
AKXIIOLURNATOC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:144 °C
-
稳定性/保质期:
如果按照规定使用和储存,则不会发生分解,目前没有已知的危险反应,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:17
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.533
-
拓扑面积:44
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
安全说明:S26,S36/37/39,S45
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2926909090
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:3276
-
储存条件:请将贮藏器保持密封状态,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
| Name: | 3 5-Di(tert-butyl)-4-hydroxybenzonitrile 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1988-88-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1988-88-1 | 3,5-Di(tert-butyl)-4-hydroxybenzonitri | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1988-88-1: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 141 - 143 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H21NO
Molecular Weight: 231
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Acids, bases, oxidizing agents.
Hazardous Decomposition Products:
Hydrogen cyanide, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1988-88-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,5-Di(tert-butyl)-4-hydroxybenzonitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: NITRILES, SOLID, TOXIC, N.O.S.*
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
IMO
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
RID/ADR
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 1988-88-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1988-88-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1988-88-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-二叔丁基-4-甲基苯酚 2,6-di-tert-butyl-4-methyl-phenol 128-37-0 C15H24O 220.355 3,5-二叔丁基-4-羟基苯甲醛 3,5-Di-t-butyl-4-hydroxybenzaldehyde 1620-98-0 C15H22O2 234.338 3,5-二-叔丁基-4-羟基苯甲醛肟 3,5-di-tert-butyl-4-hydroxybenzaldehyde oxime 787-13-3 C15H23NO2 249.353 —— 3,5-di-tert-butyl-4-hydroxybenzaldehyde oxime 69230-89-3 C15H23NO2 249.353 4-溴-2,6-二叔丁基苯酚 4-bromo-2,6-di-tert-butylphenol 1139-52-2 C14H21BrO 285.224 3,5-二-叔-丁基-4-羟基苯甲酰胺 3,5-bis(1,1-dimethylethyl)-4-hydroxybenzamide 60632-18-0 C15H23NO2 249.353 —— 3,5-di-tert-butyl-4-hydroxybenzoyl chloride 40056-43-7 C15H21ClO2 268.784 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-Methoxy-3.5-di-tert-butyl-benzonitril 4917-29-7 C16H23NO 245.365 3,5-二叔丁基-4-羟基苄胺 4-(aminomethyl)-2,6-di-tert-butylphenol 724-46-9 C15H25NO 235.37 —— 3,5-bis(1,1-dimethylethyl)-4-<(2-methoxyethoxy)methoxy>benzonitrile 130116-73-3 C19H29NO3 319.444 3-溴-4-羟基-5-叔丁基-苯甲腈 3-bromo-5-tert-butyl-4-hydroxy-benzonitrile 4910-06-9 C11H12BrNO 254.126 3,5-二叔丁基-4-羟基苯甲酸 3,5-Di-tert-butyl-4-hydroxybenzoic acid 1421-49-4 C15H22O3 250.338 3,5-二-叔-丁基-4-羟基苯甲酰胺 3,5-bis(1,1-dimethylethyl)-4-hydroxybenzamide 60632-18-0 C15H23NO2 249.353 3,5-二叔丁基-4-羟基苯甲酸甲酯 methyl 3,5-di-tert-butyl-4-hydroxybenzoate 2511-22-0 C16H24O3 264.365 3,5-二-叔-丁基-4-羟基苯甲酸乙酯 ethyl 3,5-di-tert-butyl-4-hydroxybenzoate 1620-64-0 C17H26O3 278.392 苯甲亚胺酸,3,5-二(1,1-二甲基乙基)-4-羟基-,乙基酯 ethyl iminoester of 4-hydroxy-3,5-di-tert-butylbenzoic acid 105648-45-1 C17H27NO2 277.407 —— 4-Hydroxy-3.5-di-tert-butyl-benzoesaeure-propylester 71579-48-1 C18H28O3 292.419 —— tert-butyl 3,5-di-tert-butyl-4-hydroxybenzoate 51517-24-9 C19H30O3 306.445 四叔丁基联苯酚 4,4'-dihydroxy-3,3',5,5'-tetra-tert-butylbiphenyl 128-38-1 C28H42O2 410.64 - 1
- 2
反应信息
-
作为反应物:描述:3,5-二叔丁基-4-羟基苯甲腈 以50%的产率得到2,6-Bis(1,1-dimethylethyl)-4-(3,4,8,9-tetrahydro-6-methoxy-3,3,8,8-tetramethylfuro[2,3-h]isoquinolin-1-yl)phenol hydrochloride参考文献:名称:FUROISOQUINOLINE DERIVATIVES, PROCESS FOR PRODUCING THE SAME AND USE THEREOF摘要:公开号:EP1270577B1
-
作为产物:描述:参考文献:名称:Rearrangement fragmentation of aldoxime to isocyanide摘要:DOI:10.1007/bf01457806
-
作为试剂:描述:三氯乙腈 在 aluminum tri-bromide 、 3,5-二叔丁基-4-羟基苯甲腈 作用下, 以 乙醚 为溶剂, 以27%的产率得到6-<3,5-di(tert-butyl)-4-hydroxyphenyl>-2,4-bis(trichloromethyl)-sym-trazene参考文献:名称:Synthesis and properties of sym-triazine derivatives. 8. Synthesis of amino and alkoxy derivatives of sym-triazine containing fragments of a sterically-hindered phenol from 2,4-bis(trichloromethyl)-sym-triazines摘要:DOI:10.1007/bf00755699
文献信息
-
Crystal Engineering and Substituent Effect of Hindered Phenols for SHG Materials作者:Koichi Takagi、Akira Mizuno、Michiru Kubata、Akira Mizoguchi、Masaru Furushow、Masaru MatsuokaDOI:10.1246/cl.1992.1743日期:1992.92,6-Di-tert-butylphenol moiety was found to be effective to control molecular packing in crystals for SHG materials. Many derivatives having compact acceptor group at 4-position of hindered phenols showed SHG responses depending on the electron withdrawing effect of an acceptor. The X-ray structural analyses revealed that molecular packing was controlled both by the steric requirements and the intermolecular hydrogen bonding.研究发现,含有2,6-二叔丁基苯酚部分的化合物能够有效控制SHG材料的晶体分子堆积。许多在受阻酚4位具有紧凑受体基团的衍生物,其SHG响应取决于受体的吸电子效应。X射线结构分析揭示,分子堆积既受到空间位阻要求的控制,也受到分子间氢键的影响。
-
Reaction of superoxo Co(III) complex with stable phenoxy radicals作者:A. Nishinaga、H. Tomita、T. MatsuuraDOI:10.1016/s0040-4039(00)78701-6日期:1980.1A typical superoxo complex [Co(CN)5O2][Ph3PNPPh3]3 combines with stable phenoxy radicals in CH2Cl2 leading to selective formation of peroxy--quinols except for 2,4,6-tri--butylphenoxy radical, representing radical reactivity of the complex.
-
Oxidation of 2,6-di-tert-butylphenol by tetrapyridyl oxoiron(IV) complex作者:Dóra Lakk-Bogáth、Gábor Speier、József KaizerDOI:10.1016/j.poly.2018.02.015日期:2018.5(S = 1) oxoiron(IV) complex, [FeIV(O)(asN4Py)] (2) (asN4Py = N,N-bis(2-pyridylmethyl)-1,2-di(2-pyridyl)ethylamine), has been investigated in the oxidation reaction of 2,6-di-tert-butylphenol derivatives. Detailed kinetic, and mechanistic studies (kinetic isotope effect (KIE) of 4.52, and Hammett correlation with ρ = −1.83), lead to the conclusion that the rate-determining step in this reaction involves direct
-
Phenol Oxidation by a Nickel(III)–Fluoride Complex: Exploring the Influence of the Proton Accepting Ligand in PCET Oxidation作者:Prasenjit Mondal、Aidan R. McDonaldDOI:10.1002/chem.202002135日期:2020.8.6transfer/electron transfer (PT/ET) reaction mechanism. The analogous complexes [NiIII(Z)(L)] (Z=Cl, OCO2H, O2CCH3, ONO2) reacted with all phenols through a HAT mechanism. We explore the reason for a change in mechanism with the highly basic fluoride ligand in 2 . Complex 2 was also found to react one to two orders of magnitude faster than the corresponding analogous [NiIII(Z)(L)] complexes. This was ascribed为了深入了解H +受体末端配体在高价氧化剂介导的质子偶联电子传递(PCET)反应中的影响,使用了高价氟化镍络合物的反应性[Ni III(F)(L) ](2,L = N,N'-(2,6-二甲基苯基)-2,6-吡啶氨基甲酸酯)的研究。对这些反应的动力学数据(Evans-Polanyi,Hammett和Marcus图以及KIE测量)和形成的产物进行分析,结果表明2与富电子酚通过氢原子转移(HAT或PCET协同作用)机理以及与电子反应通过逐步质子转移/电子转移(PT / ET)反应机理获得的不良酚。类似物[Ni III(Z)(L)](Z = Cl,OCO 2 H,O 2 CCH 3,ONO 2)通过HAT机理与所有酚反应。我们探索了在2中具有高碱性氟化物配体的机理发生变化的原因。还发现,配合物2的反应比相应的类似[Ni III(Z)(L)]配合物快一到两个数量级。这归因于与HF相关的高键解离自由能值(135
-
Synthesis and pharmacological evaluation of imidazoline sites I1 and I2 selective ligands作者:Maria Anastassiadou、Saı̈da Danoun、Louis Crane、Geneviève Baziard-Mouysset、Marc Payard、Daniel-Henri Caignard、Marie-Claire Rettori、Pierre RenardDOI:10.1016/s0968-0896(00)00280-7日期:2001.3heterocyclic-imidazoline compounds have been prepared and evaluated in vitro as imidazoline sites (I1 and I2) and alpha-adrenergic (alpha1 and alpha2) receptor ligands. Their pKi values indicate that linkage of the imidazoline moiety at the 2-position with an aromatic substituent dramatically decreases alpha-adrenergic affinity. I1 sites are more accessible by phenyl imidazolines substituted by a methyl or a methoxy
表征谱图
-
氢谱1HNMR
-
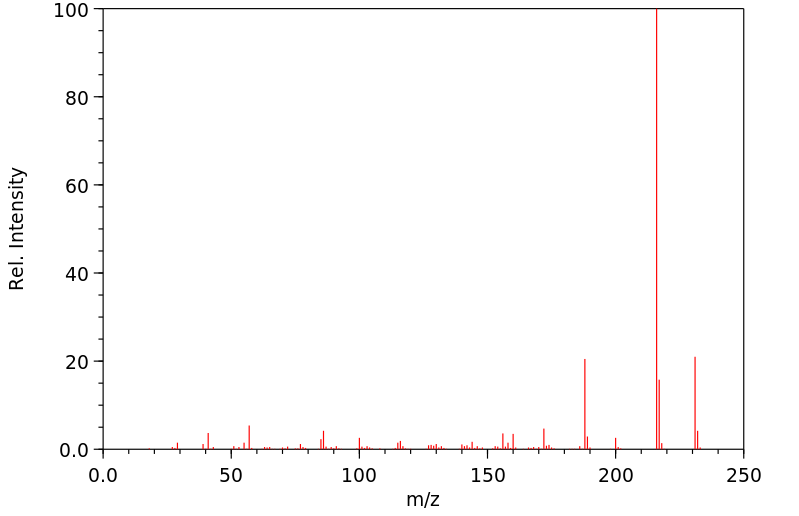
质谱MS
-
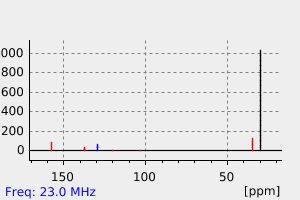
碳谱13CNMR
-
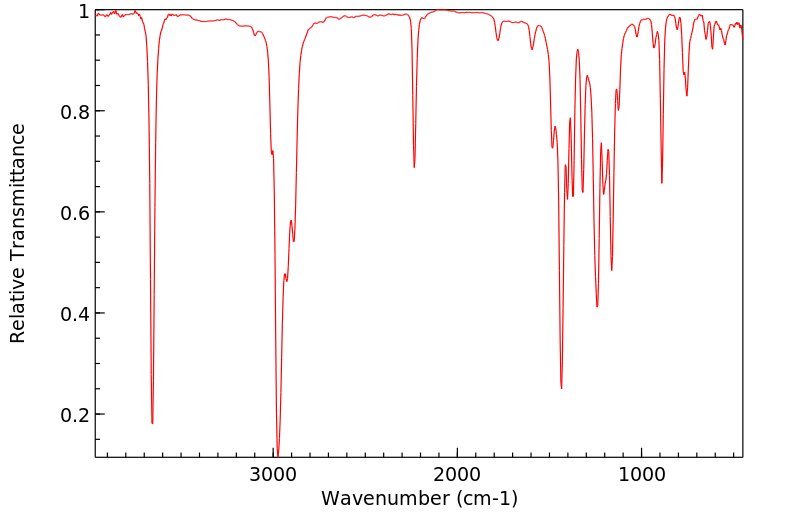
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫