3,5-二甲基溴苄 | 27129-86-8
中文名称
3,5-二甲基溴苄
中文别名
2,3-二甲基溴苄;3,5-二甲基苯甲基溴;3,5-二甲基苄基溴
英文名称
1-bromomethyl-3,5-dimethylbenzene
英文别名
3,5-Dimethylbenzyl bromide;1-(bromomethyl)-3,5-dimethylbenzene
CAS
27129-86-8
化学式
C9H11Br
mdl
MFCD00013539
分子量
199.09
InChiKey
QXDHXCVJGBTQMK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37-39 °C (lit.)
-
沸点:101-103 °C (8 mmHg)
-
密度:1.2949 (estimate)
-
闪点:109-110°C/14mm
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:8
-
安全说明:S26,S36/37/39,S45
-
危险品运输编号:3265
-
海关编码:29036990
-
危险类别:8
-
危险品标志:C
-
危险类别码:R34
-
包装等级:III
-
WGK Germany:3
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H314
-
储存条件:将贮藏器密封,并将其放入一个紧密容器中,存放在阴凉、干燥的地方。
SDS
| Name: | 3 5-Dimethylbenzyl bromide Material Safety Data Sheet |
| Synonym: | |
| CAS: | 27129-86-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 27129-86-8 | 3,5-Dimethylbenzyl bromide | 100 | unlisted |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.
Potential Health Effects
Eye:
Causes eye burns. Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin burns.
Ingestion:
Causes gastrointestinal tract burns.
Inhalation:
Causes chemical burns to the respiratory tract. Inhalation may be fatal as a result of spasm, inflammation, edema of the larynx and bronchi, chemical pneumonitis and pulmonary edema. May cause burning sensation, coughing, wheezing, laryngitis, shortness of breath, headache, nausea, and vomiting.
Chronic:
Chronic exposure may cause effects similar to those of acute exposure.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Avoid runoff into storm sewers and ditches which lead to waterways.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 27129-86-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 101 - 103 deg C @ 8 mmHg
Freezing/Melting Point: 38 - 40 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H11Br
Molecular Weight: 199.09
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents, bases, alcohols, amines, steel.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 27129-86-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,5-Dimethylbenzyl bromide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, N.O.S.*
Hazard Class: 8
UN Number: 1760
Packing Group: II
IMO
Shipping Name: CORROSIVE LIQUID, N.O.S.
Hazard Class: 8
UN Number: 1760
Packing Group: II
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 27129-86-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 27129-86-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 27129-86-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 均三甲苯 1,3,5-trimethyl-benzene 108-67-8 C9H12 120.194 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,5-二(溴甲基)甲苯 3,5-bis(bromomethyl)toluene 19294-04-3 C9H10Br2 277.986 —— 1,4-Bis(bromomethyl)-2,6-dimethylbenzene 148873-79-4 C10H12Br2 292.013 均三甲苯 1,3,5-trimethyl-benzene 108-67-8 C9H12 120.194
反应信息
-
作为反应物:参考文献:名称:惰性碳-硫键的无配体镍催化还原裂解摘要:提出了在无配体条件下C(sp 2)–和C(sp 3)–SMe键的催化还原裂解。该方法的特点是适用范围广,化学选择性高,包括具有挑战性的底物组合,可设计正交和位点选择性方法。DOI:10.1021/ol2033306
-
作为产物:描述:参考文献:名称:使用压电材料作为氧化还原催化剂对未活化的烯烃和炔烃进行机械化学邻位二溴化摘要:机械氧化还原化学是机械力和化学反应交叉领域的一个快速发展的领域。在此,我们报道了使用压电材料(Li 2 TiO 3 )作为氧化还原催化剂的不饱和烃的邻位二溴化。此外,该反应可以有效地放大至10mmol,并且在室温下在空气气氛下进行,无需溶剂或外部还原剂,并且Li 2 TiO 3可以多次重复使用而不改变结构。DOI:10.1021/acs.orglett.4c01077
文献信息
-
Discovery of Dipeptide-Derived Catalysts for the Enantioselective Addition of Dimethylzinc to Aldehydes作者:Seock Yong Kang、Yong Sun ParkDOI:10.1002/ejoc.201200063日期:2012.3new class of modular chiral catalysts derived from various amino acid-L-Pro dipeptides was prepared, and the catalysts were tested for their ability to catalyze the enantioselective addition of dimethylzinc to aromatic aldehydes. Dipeptides derived from L-Asp-L-Pro were identified as effective catalysts for the addition at room temperature with up to 97:3 er and 95 % yield.
-
Synthesis of Phenanthridines through Palladium-Catalyzed Cascade Reaction of 2-Halo-<i>N</i>-Ms-arylamines with Benzyl Halides/Sulfonates作者:Si-Yi Yang、Wen-Yong Han、Ding-Lei Zhang、Xiao-Jian Zhou、Mei Bai、Bao-Dong Cui、Nan-Wei Wan、Wei-Cheng Yuan、Yong-Zheng ChenDOI:10.1002/ejoc.201601608日期:2017.2.3An efficient palladium-catalyzed nucleophilic substitution/C–H activation/aromatization cascade reaction between readily available 2-halo-N-Ms-arylamines (Ms = methanesulfonyl) and benzyl halides/sulfonates has been described. A wide variety of phenanthridines were synthesized in a one-pot fashion in moderate to high yields (37–86 %). Notably, this method provides a straightforward, facile approach
-
Sonogashira cross-coupling reaction catalysed by mixed NHC-Pd-PPh 3 complexes under copper free conditions作者:Nedra Touj、Sedat Yaşar、Namık Özdemir、Naceur Hamdi、İsmail ÖzdemirDOI:10.1016/j.jorganchem.2018.01.017日期:2018.4characterised by NMR, HRMS, elemental analysis and X-ray crystallography for complex 3b. These complexes were applied to Sonogashira cross-coupling reactions between aryl bromides and phenylacetylene in DMF at 80 °C. All palladium complexes were stable and showed high catalytic activity in Sonogashira reactions at low catalyst loading and ambient reaction conditions.
-
Synthesis, spectroscopic properties and biological activity of new Cu(I) N-Heterocyclic carbene complexes作者:N. Touj、A. Chakchouk-Mtibaa、L. Mansour、A.H. Harrath、J. Al-Tamimi、L. Mellouli、I. Özdemir、S. Yasar、N. HamdiDOI:10.1016/j.molstruc.2018.12.093日期:2019.4Abstract New benzimidazolium salts (2) and their copper N-heterocyclic carbene complexes (3–4) were designed, synthesized and structurally characterized by NMR (1H and 13C), IR, HRMS and elemental analysis. All obtained complexes especially compounds 3d and 3e, presented significant inhibitory activity against the tested food-borne pathogens and clinical microorganisms. The compound 3b presents against摘要 设计、合成了新的苯并咪唑鎓盐 (2) 及其铜 N-杂环卡宾配合物 (3-4),并通过 NMR(1H 和 13C)、IR、HRMS 和元素分析对其进行了结构表征。所有获得的复合物,尤其是化合物 3d 和 3e,对所测试的食源性病原体和临床微生物均具有显着的抑制活性。化合物3b对金黄色葡萄球菌的MIC值为0.0195mg/ml,与用作标准的氨苄青霉素(0.0195mg/ml)非常相似。化合物 3a 和 3b 从 0.5 毫克/毫升的浓度开始,表现出与所用的两种对照丁基化羟基甲苯 (BHT) 和没食子酸 (GA) 相同的清除活性。关于乙酰胆碱酯酶抑制活性 (AChEI),化合物 3e 表现出有趣的 AChEI 活性,抑制率为 32.80%。
-
Ru(<scp>ii</scp>)–N-heterocyclic carbene complexes: synthesis, characterization, transfer hydrogenation reactions and biological determination作者:Lamia Boubakri、A. Chakchouk-Mtibaa、Abdullah S. Al-Ayed、L. Mansour、Nael Abutaha、Abdel Halim Harrath、L. Mellouli、I. Özdemir、S. Yasar、Naceur HamdiDOI:10.1039/c9ra05605j日期:——respectively 70% and 90%. In addition, these two Ru–NHC complexes exhibited antifungal activity against Candida albicans. Investigation of the anti-acetylcholinesterase activity of the studied complexes showed that compounds 3a, 3b, 3d and 3e exhibited good activity at 100 μg ml−1 and product 3d is the most active. In a cytotoxicity study the complexes 3 were evaluated against two human cancer cell lines MDA-MB-231在Ar条件下,银( I ) N-杂环卡宾配合物与[RuCl 2 ( p -cymene)] 2在二氯甲烷中发生金属转移反应,成功合成了一系列具有N-杂环卡宾配体的钌( II )配合物。所有新化合物均通过光谱和分析方法进行了表征。发现这些钌( II )-NHC配合物是在KOH作为助催化剂的情况下使用2-丙醇作为氢源进行酮转移氢化的有效预催化剂。钌 N-杂环卡宾配合物3a-f的抗菌活性用圆盘扩散法对革兰氏阳性菌和革兰氏阴性菌进行测定。化合物3d对用作指示细胞的六种细菌中的五种具有潜在的抗菌活性。产品3e抑制了所有六种测试微生物的生长。此外,使用 2,2-diphenyl-1-picrylhydrazyl (DPPH) 和 2,2'-azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) 作为试剂对这些配合物3a-f的抗氧化活性测定表明,化合物3b和3d具有
表征谱图
-
氢谱1HNMR
-
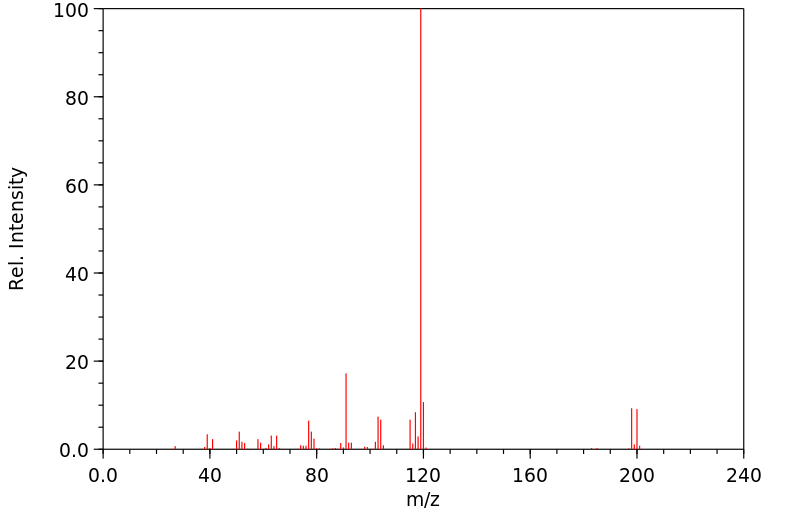
质谱MS
-
碳谱13CNMR
-
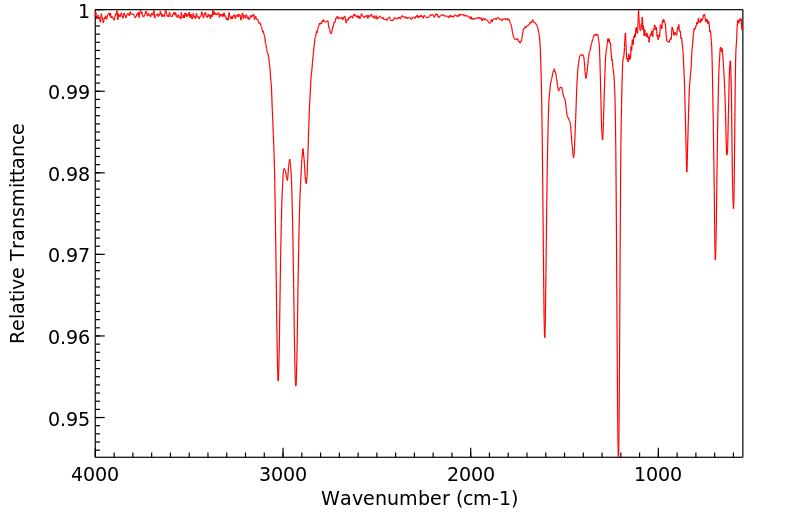
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫