3,5-辛二炔 | 16387-70-5
中文名称
3,5-辛二炔
中文别名
——
英文名称
octa-3,5-diyne
英文别名
3,5-octadiyne
CAS
16387-70-5
化学式
C8H10
mdl
MFCD00041633
分子量
106.167
InChiKey
LILZEAJBVQOINI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:27°C (estimate)
-
沸点:164 °C
-
密度:0.81
-
保留指数:972
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:8
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
Section I.Chemical Product and Company Identification
Chemical Name 3,5-Octadiyne
Portland OR
Synonym Not available.
Chemical Formula C8H10
CAS Number 16387-70-5
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
3,5-Octadiyne 16387-70-5 Min. Not available. Not available.
98.0%(GC)
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact
In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
Inhalation
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the
toxic material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability Flammable.
Flash Points Flammable Limits Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2).
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
Flammable liquid.
and Instructions SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use alcohol foam, water spray or fog. Cool containing vessels with water jet in order to prevent pressure
build-up, autoignition or explosion. Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
3,5-Octadiyne
Section VI. Accidental Release Measures
Spill Cleanup Flammable liquid.
Instructions Keep away from heat. Mechanical exhaust required. Stop leak if without risk. Absorb with DRY earth, sand or other
non-combustible material. DO NOT touch spilled material. Prevent entry into sewers, basements or confined areas; dike
if needed. Consult federal, state, and/or local authorities for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage FLAMMABLE. Keep away from heat. Mechanical exhaust required. Avoid excessive heat and light. Do not breathe
gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient;
consult a specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Liquid. (Clear, Colorless.) Not available.
0.81(water=1)
Specific Gravity
Molecular Weight 106.16 Partition Coefficient Not available.
Boiling Point 164°C (327.2°F) Vapor Pressure Not available.
Melting Point Not available. Vapor Density Not available.
Not available. Volatility Not available.
Refractive Index
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Eye Contact. Ingestion. Inhalation.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
Continued on Next Page
3,5-Octadiyne
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification DOT Class 3: Flammable liquid
PIN Number
Proper Shipping Name
Hydrocarbons, liquid, n.o.s.
Packing Group (PG) III
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements R10- Flammable.
R18- In use, may form flammable/explosive vapor-air mixture.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:活化锌粉对区域和立体特异性还原共轭和非共轭三键摘要:在无水乙醇中,用1,2-二溴乙烷活化的锌粉,以及依次用二溴乙烷和溴化铜(I)活化的锌粉,进行了多种炔属衍生物的区域还原和立体特异性还原。与第二种试剂相比,第一种试剂的功能更弱,选择性更高。DOI:10.1039/c39840000735
-
作为产物:描述:1-丁炔 在 吡啶 、 氧气 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 、 copper(I) bromide 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 以90%的产率得到3,5-辛二炔参考文献:名称:A Procedure for the Oxidative “Dimerization” of Aliphatic 1-Alkynes摘要:Aliphatic 1-alkynes RC = CH (R = alkyl) are readily converted into diynes R = CC = CR, by introducing oxygen into a mixture of the acetylene and pyridine using copper(I)bromide as a catalyst and 1,8-diazabicyclo[5.4.0] undec-7-ene as a co-catalyst.DOI:10.1080/00397919108020801
文献信息
-
Rhodium-Catalyzed Oxidative Annulation of (2-Arylphenyl)boronic Acids with Alkynes: Selective Synthesis of Phenanthrene Derivatives作者:Tetsuya Satoh、Masahiro Miura、Tomoya Nagata、Yuji NishiiDOI:10.1055/s-0035-1561940日期:——A rhodium-catalyzed annulative coupling of (2-arylphenyl)boronic acids with alkynes has been developed for the facile construction of phenanthrene frameworks. The reaction proceeded without external bases, and dioxygen worked as a terminal oxidant. Deuterium-labeling experiments indicated the involvement of five-membered rhodacycle intermediates.
-
Synthesis of 1<i>H</i>-Inden-1-ol Derivatives via Rhodium-catalyzed Annulation of<i>o</i>-Acylphenylboronic Acids with Alkynes作者:Takanori Matsuda、Masaomi Makino、Masahiro MurakamiDOI:10.1246/cl.2005.1416日期:2005.10A rhodium-catalyzed annulation reaction of o-formylphenylboronic acid with alkynes occurred regioselectively at room temperature to give substituted 1H-inden-1-ol derivatives. o-Acetylphenylboronic acid also underwent the annulation reaction at 80 °C to afford 1-methyl-1H-inden-1-ols.
-
Oxidative addition of 1,3-diynes at triosmium clusters with cleavage of the central carbon–carbon bond: X-ray crystal structure of [Os<sub>3</sub>(µ<sub>3</sub>,η<sup>2</sup>-C<sub>2</sub>Ph)(µ-C<sub>2</sub>Ph)(CO)<sub>9</sub>] derived from 1,4-diphenylbuta-1,3-diyne作者:Antony J. Deeming、Mark S. B. Felix、Paul A. Bates、Michael B. HursthouseDOI:10.1039/c39870000461日期:——The diyne clusters [Os3(µ3,η2-RCCCCR)(CO)10](R = Me, Et, Ph, But, SiMe3) in hydrocarbon solvent at 120 °C decarbonylate to give the nonacarbonyl clusters [Os3(µ3,η2-C2R)(µ-C2R)(CO)9] when R = Ph, But, or SiMe3, [Os3H(µ3,σ,η2,η2-EtCC–CCCHMe)(CO)9] when R = Et, and [Os3(C4Me2)(CO)9] of known structure when R = Me; the µ-C2Ph ligand in [Os3(µ3, η2-C2Ph)(µ-C2Ph)(CO)9](X-ray structure) is unusual in bridging
-
Chromium-Mediated Synthesis of Polycyclic Aromatic Compounds from Halobiaryls作者:Ken-ichiro Kanno、Yuanhong Liu、Atsushi Iesato、Kiyohiko Nakajima、Tamotsu TakahashiDOI:10.1021/ol052214x日期:2005.11.1[reaction: see text] Reaction of 2,2'-dihalobiphenyl with butyllithium followed by the addition of chromium(III) chloride and alkynes afforded the corresponding phenanthrene derivatives via formal [4 + 2] cycloaddition. A variety of alkynes could be used for this reaction, such as alkyl, aryl, silyl, and alkoxycarbonyl alkynes. Repetitive process of the reaction gave more extended polycyclic compounds
-
Carbon−Carbon Bond Formation Reaction of Zirconacyclopentadienes with Alkynes in the Presence of Ni(II)-complexes作者:Tamotsu Takahashi、Fu-Yu Tsai、Yanzhong Li、Kiyohiko Nakajima、Martin KotoraDOI:10.1021/ja990750c日期:1999.12.1tetrahydroisoquinoline derivatives in good to high yields. This procedure was also used for the selective preparation of benzene derivatives from three different alkynes. The use of trimethylsilyl-substituted alkyne as the first, second or third alkyne afforded desilylated benzene derivatives. The reaction of zirconacyclopentadienes with allenes gave benzene derivatives as a mixture of two isomers.
表征谱图
-
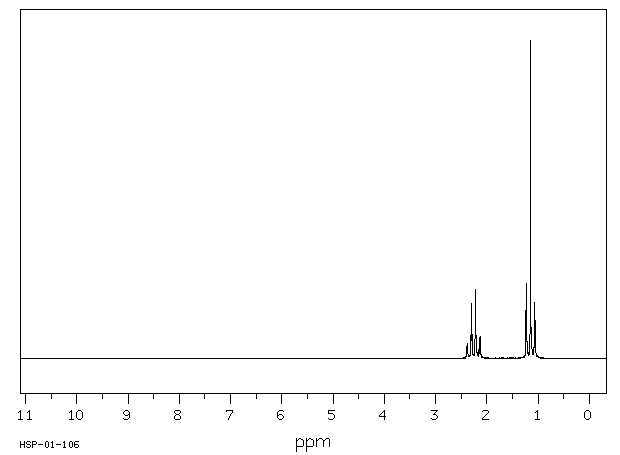
氢谱1HNMR
-
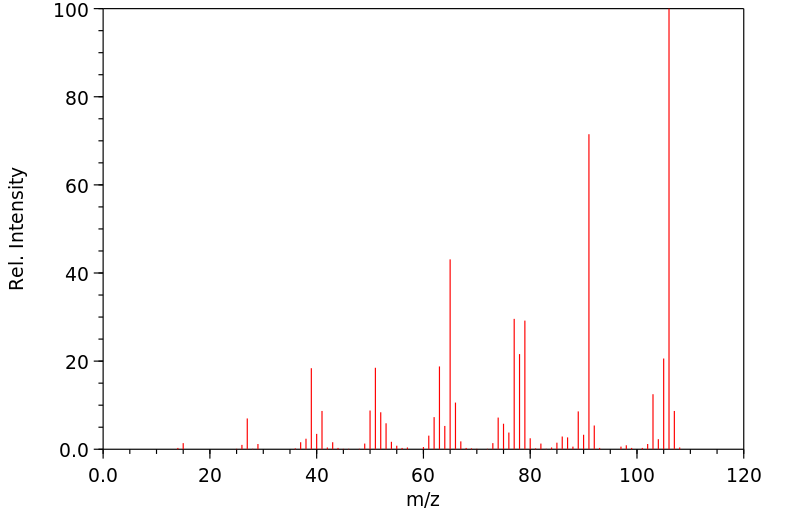
质谱MS
-
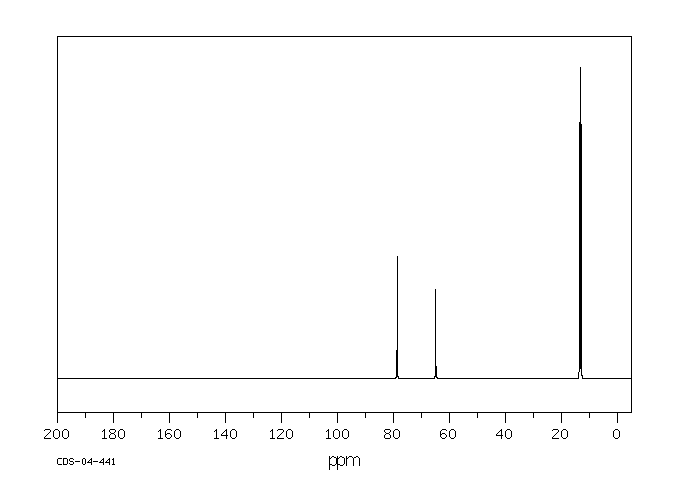
碳谱13CNMR
-
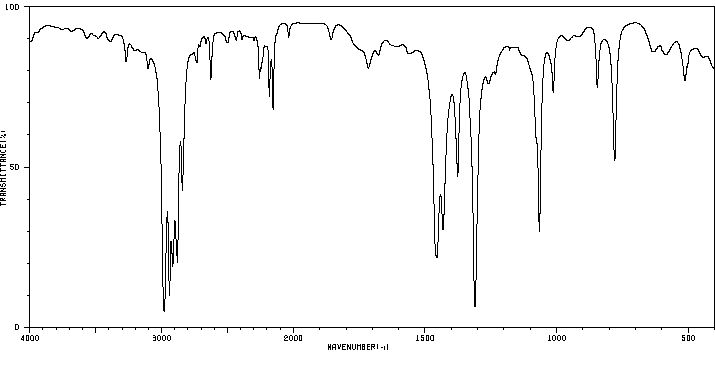
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-