3-乙酰基-2,4-二甲基-5-乙氧羰基吡咯 | 2386-26-7
中文名称
3-乙酰基-2,4-二甲基-5-乙氧羰基吡咯
中文别名
4-乙酰基-3,5-二甲基-1H-吡咯-2-甲酸乙酯;2,4-二甲基-3-乙酰基-5-乙酰氧基吡咯;3-乙酰-2,4-二甲基-5乙氧羰基吡咯;2,4-二甲基-3-乙酰基-5-乙酸基吡咯
英文名称
ethyl 4-acetyl-3,5-dimethylpyrrole-2-carboxylate
英文别名
ethyl 4-acetyl-3,5-dimethyl-1H-pyrrole-2-carboxylate
CAS
2386-26-7
化学式
C11H15NO3
mdl
MFCD00022373
分子量
209.245
InChiKey
ALRDOFWBPAZOCW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:143 °C
-
沸点:348.6°C (rough estimate)
-
密度:1.1547 (rough estimate)
-
稳定性/保质期:
避免让氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:15
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.454
-
拓扑面积:59.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S22,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933990090
-
储存条件:请将容器密封,并存放在干燥、阴凉的地方。
SDS
| Name: | Ethyl 4-acetyl-3 5-dimethyl-1h-pyrrole-2-carboxylate 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2386-26-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2386-26-7 | Ethyl 4-acetyl-3,5-dimethyl-1H-pyrrole | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2386-26-7: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 139 - 141 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C11H15NO3
Molecular Weight: 209
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2386-26-7: UX9370900 LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 4-acetyl-3,5-dimethyl-1H-pyrrole-2-carboxylate - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 2386-26-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2386-26-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2386-26-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二甲基-1H-吡咯-2-甲酸乙酯 3,5-dimethyl-2-ethoxycarbonyl-1H-pyrrole 2199-44-2 C9H13NO2 167.208 —— 4-(1-chloro-vinyl)-3,5-dimethyl-pyrrole-2-carboxylic acid ethyl ester 65613-22-1 C11H14ClNO2 227.691 4-碘-3,5-二甲基-1H-吡咯-2-羧酸乙酯 ethyl 4-iodo-3,5-dimethyl-1H-pyrrole-2-carboxylate 5408-08-2 C9H12INO2 293.104 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-乙酰基-5-甲酰基-3-甲基-1H-吡咯-2-羧酸乙酯 2-Formyl-4-methyl-3-acetyl-pyrrol-5-carbonsaeureethylester 33423-48-2 C11H13NO4 223.229 —— 4-acetyl-5-methoxymethyl-3-methyl-pyrrole-2-carboxylic acid ethyl ester 50995-33-0 C12H17NO4 239.271 4-乙酰基-5-[(乙酰基氧基)甲基]-3-甲基-1H-吡咯-2-羧酸乙酯 ethyl 5-acetoxymethyl-4-acetyl-3-methylpyrrole-2-carboxylate 143583-56-6 C13H17NO5 267.282 —— 4-acetyl-5-chloromethyl-3-methyl-pyrrole-2-carboxylic acid ethyl ester 2386-35-8 C11H14ClNO3 243.69 —— benzyl 4-acetyl-3,5-dimethylpyrrole-2-carboxylate 2386-27-8 C16H17NO3 271.316 4-乙酰基-3,5-二甲基-1H-吡咯-2-羧酸 4-acetyl-3,5-dimethyl-1H-pyrrole-2-carboxylic acid 2386-28-9 C9H11NO3 181.191 4-乙基-3,5-二甲基-1H-吡咯-2-甲酸乙酯 ethyl 3,5-dimethyl-4-ethylpyrrole-2-carboxylate 2199-47-5 C11H17NO2 195.261 —— ethyl-3,5-dimethyl-4-(3-oxo-3-phenyl- propenyl)-1H-pyrrole-2-carboxylate 4267-48-5 C18H19NO3 297.354 —— ethyl 3,5-dimethyl-4-(3-oxo-3-phenylpropenyl)-1H-pyrrole-2-carboxylate —— C18H19NO3 297.354 —— <2,4-Dimethyl-5-ethoxycarbonyl-pyrryl-(3)>-glyoxylsaeure 21898-55-5 C11H13NO5 239.228 —— (E)-ethyl-4–(3-(3–methoxyphenyl)acryloyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate —— C19H21NO4 327.38 —— 5-bromomethyl-4-ethyl-3-methyl-pyrrole-2-carboxylic acid ethyl ester 4789-44-0 C11H16BrNO2 274.158 —— 3-Acetyl-5-ethoxycarbonyl-1,2,4-trimethylpyrrole 40861-85-6 C12H17NO3 223.272 —— 4-ethyl-1,3,5-trimethyl-pyrrole-2-carboxylic acid ethyl ester 99663-15-7 C12H19NO2 209.288 —— 3-Acetyl-5-carboxy-1,2,4-trimethylpyrrole 136558-76-4 C10H13NO3 195.218 —— ethyl 4-ethynyl-3,5-dimethylpyrrole-2-carboxylate 52649-03-3 C11H13NO2 191.23 4-乙酰基-3,5-二甲基-1H-吡咯-2-甲醛 4-Acetyl-3,5-dimethyl-1H-pyrrole-2-carbaldehyde 2386-30-3 C9H11NO2 165.192 —— 3-(3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)indole 143583-55-5 C19H20N2O3 324.379 —— 4,3'-diacetyl-5,5'-dibenzyloxycarbonyl-3,4'-dimethyl-2,2'-dipyrrylmethane 1063631-12-8 C31H30N2O6 526.589 —— 3-acetyl-5-[(3-acetyl-5-carboxy-4-methyl-1H-pyrrol-2-yl)methyl]-4-methyl-1H-pyrrole-2-carboxylic acid 1063631-13-9 C17H18N2O6 346.34 —— 3-(3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)benzothiophene 143772-78-5 C19H19NO3S 341.431 —— 2-(3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)benzofuran 143772-75-2 C19H19NO4 325.364 3,5-二甲基-1H-吡咯-2-甲酸乙酯 3,5-dimethyl-2-ethoxycarbonyl-1H-pyrrole 2199-44-2 C9H13NO2 167.208 —— 4-(1-chloro-vinyl)-3,5-dimethyl-pyrrole-2-carboxylic acid ethyl ester 65613-22-1 C11H14ClNO2 227.691 5-甲酰基-3-甲基-1H-吡咯-2,4-二甲酸 2-formyl-4-methylpyrrole-3,5-dicarboxylic acid 79754-38-4 C8H7NO5 197.147 乙基1,3,5-三甲基-1H-吡咯-2-羧酸酯 ethyl 1,3,5-trimethylpyrrole-2-carboxylate 55770-79-1 C10H15NO2 181.235 —— 2,3-bis(3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)benzothiophene 143772-79-6 C30H32N2O6S 548.66 —— 3-(1'-Chlorovinyl)-5-ethoxycarbonyl-1,2,4-trimethylpyrrole 136558-75-3 C12H16ClNO2 241.718 —— 2,3-bis(3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)benzofuran 143772-76-3 C30H32N2O7 532.593 - 1
- 2
- 3
反应信息
-
作为反应物:描述:3-乙酰基-2,4-二甲基-5-乙氧羰基吡咯 在 磺酰氯 、 Montmorillonite K10 clay 作用下, 以 乙醚 、 1,2-二氯乙烷 为溶剂, 反应 4.0h, 生成 3-(3'-acetyl-5'-ethoxycarbonyl-4'-methylpyrrol-2'-ylmethyl)indole参考文献:名称:Chunchatprasert, Laddawan; Rao, K. R. Nagaraja; Shannon, Patrick V. R., Journal of the Chemical Society. Perkin transactions I, 1992, # 14, p. 1779 - 1784摘要:DOI:
-
作为产物:参考文献:名称:KOZHICH D. T.; AKIMENKO L. V.; MIRONOV A. F.; EVSTIGNEEVA R. P., ZH. ORGAN. XIMII, 1977, 13, HO 12, 2604-2608摘要:DOI:
文献信息
-
Total Synthesis of Hematoporphyrin and Protoporphyrin: A Conceptually New Approach作者:Pierre Martin、Markus Mueller、Dietmar Flubacher、Andreas Boudier、Hans-Ulrich Blaser、Dirk SpielvogelDOI:10.1021/op100036c日期:2010.7.16The total synthesis of protoporphyrin IX and its disodium salt using a new alternative method to the classical MacDonald condensation is reported. The key step is the reaction of the new unsymmetrical diiodo dipyrrylmethane 1 with the known dipyrrylmethane 2. Coupling of the two fragments leads directly to porphyrin 3 without the need of an oxidizing agent. The new methodology is well suited for the
-
Process For Preparing Porphyrin Derivatives, Such As Protoporphyrin (IX) And Synthesis Intermediates申请人:Martin Pierre公开号:US20080242857A1公开(公告)日:2008-10-02The present invention relates to a process for preparing a porphyrin of formula (I), optionally in the form of a salt with an alkali metal and/or in the form of a metal complex: in which: R and R′ are as defined in claim 1 , comprising: a step of condensation, in an acidic medium, between a dipyrromethane of formula (II): in which R′b is as defined above for (I), and a dipyrromethane of formula (III): in which R″ is as defined in claim 1 , and also the compounds of formula (III).
-
Total Synthesis of Hematoporphyrin and Protoporphyrin; a Conceptually New Approach作者:Pierre Martin、Markus Müller、Dietmar Flubacher、Andreas Boudier、Dirk SpielvogelDOI:10.2533/chimia.2013.204日期:——
The total synthesis of protoporphyrin IX and its disodium salt using a new alternative method to the classical MacDonald condensation is reported. The key step is the reaction of the new unsymmetrical diiodo dipyrrylmethane 1 with the known dipyrrylmethane 2. Coupling of the two fragments leads directly to porphyrin 3 without the need of an oxidizing agent. The new methodology is well suited for the synthesis of protoporphyrin IX derivatives on a multi-100 g scale in good quality without the need for chromatography. Furthermore, these preparations are completely free of any contaminant of animal origin, which represents a real improvement in the manufacturing of protoporphyrin IX derivatives.
-
Design synthesis and anti-proliferative activity of some new coumarin substituted hydrazide–hydrazone derivatives作者:Nongnaphat Duangdee、Wiratchanee Mahavorasirikul、Saisuree PrateeptongkumDOI:10.1007/s12039-020-01767-4日期:2020.12hydrazide–hydrazone derivatives. Unfortunately, all test compounds, as well as doxorubicin, showed no cytotoxicity toward drug-resistant cell line, Caco-2. Our preliminary results indicated that coumarin hydrazide–hydrazone derivatives could be exploited as leading structures for further anticancer-drug development. Graphic abstract Synthesis of coumarin substituted hydrazide-hydrazone derivatives摘要 设计,合成和评估了一系列21种香豆素酰肼-hydr衍生物,并在体外以25μg/ mL的浓度评估48 h对肝癌(HepG2)细胞系的潜在细胞毒性作用。然后,从21种化合物中筛选出7种细胞存活率低于60%的化合物,以评估其对肝癌(HepG2),乳腺癌(SKBR-3)和人结肠癌(Caco-2)细胞的体外抗增殖活性线。在测试化合物中,5g,6d和6f对Hep-G2和SKBR-3细胞系均显示出有效的活性。更重要的是,具有4-溴苯基部分的化合物6d对IC 50表现出对Hep-G2细胞系的最佳细胞毒性活性值2.84±0.48μg/ mL,与标准阿霉素相当(IC 50 = 2.11±0.13μg/ mL)。此外, 与其他经测试的香豆素酰肼-hydr衍生物相比,具有4-甲氧基苯基部分的化合物6f对SKBR-3细胞系表现出最强的活性(IC 50 = 2.34±0.68μg/ mL)。不幸的是,所有测试
-
Discovery of 1-(5-(1H-benzo[d]imidazole-2-yl)-2,4-dimethyl-1H-pyrrol-3-yl)ethan-1-one derivatives as novel and potent bromodomain and extra-terminal (BET) inhibitors with anticancer efficacy作者:Bo Kong、Zhaohong Zhu、Hongmei Li、Qianqian Hong、Cong Wang、Yu Ma、Wan Zheng、Fei Jiang、Zhimin Zhang、Ting Ran、Yuanyuan Bian、Na Yang、Tao Lu、Jiapeng Zhu、Weifang Tang、Yadong ChenDOI:10.1016/j.ejmech.2021.113953日期:2022.1modifications led to the identification of compound 35f as the most active inhibitor of BET BRD4 with selectivity against BET family proteins. Further biological studies revealed that compound 35f can arrest the cell cycle in G0/G1 phase and induce apoptosis via decreasing the expression of c-Myc and other proteins related to cell cycle and apoptosis. More importantly, compound 35f showed favorable pharmacokinetic作为表观遗传阅读器,溴结构域和末端外结构域 (BET) 家族蛋白与组蛋白中的乙酰化赖氨酸残基结合并募集蛋白质复合物以促进转录起始和延伸。通过小分子抑制剂抑制 BET 溴结构域已成为一种有前途的癌症治疗策略。在这里,我们描述了我们为发现一系列新的 1-(5-(1 H -benzo[ d ]imidazole-2-yl)-2,4-dimethyl-1 H -pyrrol-3-yl)ethan 所做的努力-1-one 衍生物作为 BET 抑制剂。密集的结构修饰导致化合物35f被鉴定为对 BET 家族蛋白具有选择性的最活跃的 BET BRD4 抑制剂。进一步的生物学研究表明,化合物35f通过降低c-Myc等细胞周期和细胞凋亡相关蛋白的表达,使细胞周期停滞在G 0 /G 1期,诱导细胞凋亡。更重要的是,化合物35f在 MV4-11 小鼠异种移植模型中显示出良好的药代动力学特性和抗肿瘤功效,具有可接受的耐受性。这些结果表明,BET
表征谱图
-
氢谱1HNMR
-
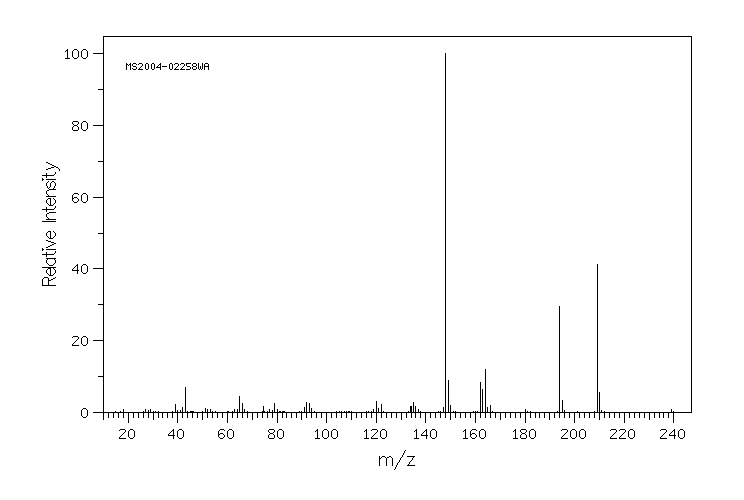
质谱MS
-
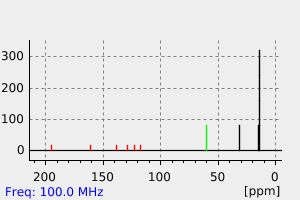
碳谱13CNMR
-
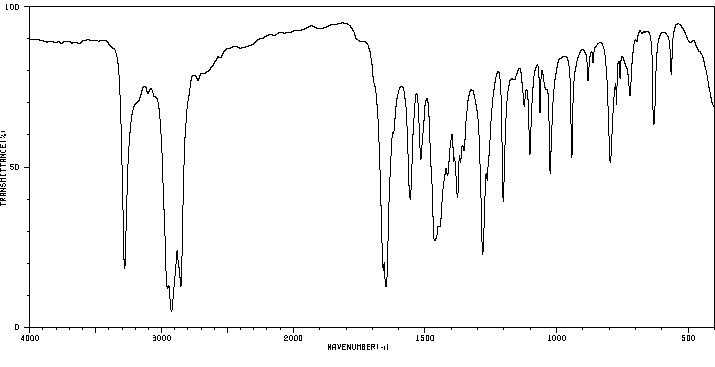
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷