盐酸氟西汀 | 56296-78-7
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:158-159°C
-
比旋光度:-0.05~+0.05°
-
闪点:9℃
-
溶解度:H2O:可溶(微溶)
-
颜色/状态:White to off-white crystalline solid
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):4.17
-
重原子数:23
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.294
-
拓扑面积:21.3
-
氢给体数:2
-
氢受体数:5
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S26,S36/37/39
-
危险类别码:R22,R41,R38
-
WGK Germany:3
-
海关编码:2922199090
-
危险品运输编号:3249
-
危险类别:6.1(b)
-
包装等级:III
-
RTECS号:UI4050000
-
危险标志:GHS05,GHS07,GHS09
-
危险性描述:H302,H318,H400
-
危险性防范说明:P273,P280,P305 + P351 + P338
-
储存条件:请将贮藏器密封保存,并存放在阴凉干燥处。务必确保工作环境具有良好的通风或排气设施。
SDS
模块 1. 化学品
1.1 产品标识符
: 氟西汀 盐酸盐
产品名称
1.2 鉴别的其他方法
(±)-N-Methyl-γ-[4-(trifluoromethyl)phenoxy]benzenepropanaminehydrochloride
Prozac®
LY-110,140hydrochloride
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
严重眼睛损伤 (类别 1)
急性水生毒性 (类别 1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H302 吞咽有害。
H318 造成严重眼损伤。
H400 对水生生物毒性极大。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P273 避免释放到环境中。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P330 漱口。
P391 收集溢出物。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: (±)-N-Methyl-γ-[4-
别名
(trifluoromethyl)phenoxy]benzenepropanaminehydrochloride
Prozac®
LY-110,140hydrochloride
: C17H18F3NO · HCl
分子式
: 345.79 g/mol
分子量
组分 浓度或浓度范围
Methyl[3-phenyl-3-[4-(trifluoromethyl)phenoxy]propyl]ammonium chloride
<=100%
化学文摘登记号(CAS 56296-78-7
No.) 260-101-2
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
症状和征兆包括头痛,眩晕,疲倦,肌肉乏力,磕睡,严重时会失去知觉。,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体, 氟化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 1.8 在 25 °C
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 452 mg/kg
皮肤刺激或腐蚀
皮肤 - 兔子 - 无皮肤刺激
眼睛刺激或腐蚀
眼睛 - 兔子 - 严重的眼睛刺激
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
生殖毒性 - 兔子 - 经口
母体效应:其他影响。
生殖毒性 - 大鼠 - 经口
对新生儿的影响:死婴。 对新生儿的影响:存活率(例如#第4天存活率每#出生成活数)。
对新生儿的影响:生长统计数据(例如体重增长的减少)。
发育毒性 - 大鼠 - 皮下的
特定发育异常:中枢神经系统。 对新生儿的影响:生长统计数据(例如体重增长的减少)。
发育毒性 - 大鼠 - 经口
特定发育异常:皮肤及皮肤附属物。
发育毒性 - 人 - 人 - 经口
特定发育异常:中枢神经系统。
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 引起眼睛灼伤。
接触后的征兆和症状
症状和征兆包括头痛,眩晕,疲倦,肌肉乏力,磕睡,严重时会失去知觉。,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: UI4050000
模块 12. 生态学资料
12.1 生态毒性
对鱼类的毒性 半数致死浓度(LC50) - 虹鳟 (红鳟鱼) - 1.57 mg/l - 96.0 h
对水蚤和其他水生无脊 半数效应浓度(EC50) - 大型蚤 (水蚤) - 0.94 mg/l - 48 h
椎动物的毒性
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
对水生生物毒性极大。
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 3077 国际海运危规: 3077 国际空运危规: 3077
14.2 联合国运输名称
欧洲陆运危规: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Methyl[3-phenyl-3-[4-
(trifluoromethyl)phenoxy]propyl]ammonium chloride)
国际海运危规: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S. (Methyl[3-phenyl-3-[4-
(trifluoromethyl)phenoxy]propyl]ammonium chloride)
国际空运危规: EnvironmeNTAlly hazardous subSTance, solid, n.o.s. (Methyl[3-phenyl-3-[4-
(trifluoromethyl)phenoxy]propyl]ammonium chloride)
14.3 运输危险类别
欧洲陆运危规: 9 国际海运危规: 9 国际空运危规: 9
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 是 国际海运危规 国际空运危规: 是
海洋污染物(是/否): 是
14.6 对使用者的特别提醒
进一步信息
危险品独立包装,液体5升以上或固体5公斤以上,每个独立包装外和独立内包装合并后的外包装上都必须有EHS
标识 (根据欧洲 ADR 法规 2.2.9.1.10, IMDG 法规 2.10.3),
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
盐酸氟西汀是全球第一个上市的选择性5-羟色胺再摄取抑制剂(SSRIs)的抗抑郁药物,商品名为“百优解” (Prozac),由美国礼来公司率先研制成功。在1986年比利时首先批准其用于抑郁症治疗后,于同年年底获得FDA在美国市场的上市许可,并以商品名Prozac成为美国销量最好的抗抑郁药。随后,氟西汀陆续进入英国、法国等西方国家市场,并在全球范围内销售。1995年4月,该药物首次在中国市场获批,商品名为百优解(后更名为百忧解),剂型为片剂和胶囊,规格为20mg。目前已有十余家国内药企获得了生产批文。
由于氟西汀是第一个上市的5-HT再摄取抑制剂类抗抑郁药物,其与传统的三环类抗抑郁剂具有不同的结构和药理机制。它疗效确切、副作用轻,在上市后即受到广泛青睐,并且销售额逐年大幅增长。自1970年代礼来公司研究开发出氟西汀以来,该药物创造了诸多辉煌,堪称一代传奇,开创了抑郁症治疗的新篇章。尽管2001年后市场有所下滑,但目前仍是抗抑郁治疗的常用药物之一。
药理作用氟西汀能阻断5-HT2A受体,并通过抑制5-HT2A受体恢复多巴胺(DA)和去甲肾上腺素(NE)的水平。同时,它还能通过5-HT2C受体解除γ-氨基丁酸(GABA)神经元对NE和DA释放的抑制作用。这些机制不仅提高突触间隙中5-HT浓度,还直接和间接恢复了DA和NE水平,具有多巴胺和去甲肾上腺素脱抑制特性(NDDI)。
应用盐酸氟西汀可用于治疗各种抑郁性精神障碍,包括轻度或重度抑郁症、双相情感性精神障碍的抑郁症、心因性抑郁及抑郁性神经症。此外,它也可用于治疗器质性疾病伴有的抑郁症状、老年期抑郁症、精神分裂症后抑郁以及强迫症、恐惧症和焦虑症状,并可作为减肥及戒烟的辅助治疗。
不良反应盐酸氟西汀的主要不良反应包括恶心、神经过敏、失眠等,这些通常在停药后会自行消失。其他常见副作用还包括头痛、焦虑、多汗、腹泻、视力模糊、口干以及性功能障碍。
抗抑郁药盐酸氟西汀是一种选择性的5-羟色胺再摄取抑制剂(SSRI),适用于治疗各种抑郁症,包括轻性和重性抑郁症、双相情感性精神障碍的抑郁症、心因性抑郁及抑郁性神经症。其作用机制是通过抑制神经元从突触间隙中摄取5-羟色胺来增加间隙中可供实际利用的这种神经递质,从而改善情感状态,治疗抑郁性精神障碍。
常见不良反应包括口干、食欲减退、恶心、失眠和乏力,少数病例可能出现焦虑或头痛。由于盐酸氟西汀半衰期较长,肝肾功能较差者或老年患者应适当减少剂量;儿童应用时应遵照医嘱;癫痫史患者、妊娠或哺乳期妇女慎用。如果出现皮疹或发热,应立即停药并进行相应处理。不宜与单胺氧化酶抑制剂(MAOIs)同时使用。
体内研究在动物成年雄性SD大鼠中,氟西汀能够扭转不可避免的冲击导致的逃避潜伏期,并且当与奥兰扎平联用时,表现出稳健、持久的细胞外多巴胺水平和去甲肾上腺素水平增加,其效果显著大于两种药物单独使用。
此外,在急性全身给药后,氟西汀产生强劲而持续增加的多巴胺和去甲肾上腺素的胞外浓度。氟西汀还能加速未成熟神经元的成熟,并增强海马齿状回的神经依赖性长时程增强(LTP)。与舍曲林、帕罗西汀、文拉法辛等相比,氟西汀在前额叶皮层中增加多巴胺和去甲肾上腺素的细胞外水平。这些体内研究表明了氟西汀对神经系统的影响及其潜在机制。
反应信息
-
作为反应物:参考文献:名称:NO-SSRIs: Nitric Oxide Chimera Drugs Incorporating a Selective Serotonin Reuptake Inhibitor摘要:Hybrid nitrate drugs have been reported to provide NO bioactivity to ameliorate side effects or to provide ancillary therapeutic activity. Hybrid nitrate selective serotonin reuptake inhibitors (NO-SSRIs) were prepared to improve the therapeutic profile of this drug class. A synthetic strategy for use of a thiocarbamate linker was developed, which in the case of NO-fluoxetine facilitated hydrolysis to fluoxetine at pH 7.4 within 7 h. In cell culture, NO-SSRIs were weak inhibitors of the serotonin transporter; however, in the forced swimming task (FST) in rats, NO-fluoxetine demonstrated classical antidepressant activity. Comparison of NO-fluoxetine, with fluoxetine, and an NO-chimera nitrate developed for Alzheimer's disease (GT-1061) were made in the step through passive avoidance (STPA) test of learning and memory in rats treated with scopolamine as an amnesic agent. Fluoxetine was inactive, whereas NO-fluoxetine and GT-1061 both restored long-term memory. GT-1061 also produced antidepressant behavior in FST. These data support the potential for NO-SSRIs to overcome the lag in onset of therapeutic action and provide cotherapy of neuropathologies concomitant with depression.DOI:10.1021/ml2000033
-
作为产物:参考文献:名称:Synthesis of 3-aminomethyl-1-propanol, a fluoxetine precursor摘要:本发明涉及一种合成氟西汀盐酸盐的方法。该方法包括通过将苯基-3-甲基氨基-1-丙烯-1-酮还原为3-甲基氨基-1-苯基-1-丙醇,使用硼氢化钠和乙酸进行合成。公开号:US20040102651A1
-
作为试剂:描述:N,N-二甲基乙酰胺 、 对氯三氟甲苯 、 N-甲基-3-苯基-3-羟基丙胺 、 sodium;hydride 在 甲苯 、 盐酸 、 盐酸氟西汀 作用下, 以 甲苯 、 水 为溶剂, 反应 1.0h, 生成 盐酸氟西汀参考文献:名称:Production of fluoxetine and new intermediates摘要:4-甲基-3-[(4-三氟甲基)苯氧基]-3-苯基丙胺(I)是通过将3-二甲氨基-1-苯基-1-丙醇(III)与卤代甲酸酯(VIII)反应,得到取代的丙基氨基甲酸酯(IX),在碱性条件下水解得到甲基氨基-1-苯基-1-丙醇(X)。然后,将甲基氨基-1-苯基-1-丙醇与4-卤苯三氟化物(XI)反应,制备出氟西汀(I)。在此过程中,还获得了某些取代的氨基甲酸酯作为中间体。公开号:US05225585A1
文献信息
-
NAPHTHALENE-BASED INHIBITORS OF ANTI-APOPTOTIC PROTEINS申请人:Pellecchia Maurizio公开号:US20090105319A1公开(公告)日:2009-04-23Methods of using apogossypol and its derivatives for treating inflammation is disclosed. Also, there is described a group of compounds having structure A, or a pharmaceutically acceptable salt, hydrate, N-oxide, or solvate thereof are provided: wherein each R is independently selected from the group consisting of H, C(O)X, C(O)NHX, NH(CO)X, SO 2 NHX, and NHSO 2 X, wherein X is selected from the group consisting of an alkyl, a substituted alkyl, an aryl, a substituted aryl, an alkylaryl, and a heterocycle. Compounds of group A may be used for treating various diseases or disorders, such as cancer.
-
Amino-substituted heterocycles, compositions thereof, and methods of treatment therewith申请人:D'Sidocky Neil R.公开号:US20080242694A1公开(公告)日:2008-10-02Provided herein are Heterocyclic Compounds having the following structure: wherein R 1 , R 2 , X, Y and Z are as defined herein, compositions comprising an effective amount of a Heterocyclic Compound and methods for treating or preventing cancer, inflammatory conditions, immunological conditions, metabolic conditions and conditions treatable or preventable by inhibition of a kinase pathway comprising administering an effective amount of a Heterocyclic Compound to a patient in need thereof.
-
[EN] HETEROCYCLIC AMIDES USEFUL AS PROTEIN MODULATORS<br/>[FR] AMIDES HÉTÉROCYCLIQUES UTILES EN TANT QUE MODULATEURS DE PROTÉINE申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2017175147A1公开(公告)日:2017-10-12Disclosed are compounds having the formula (I-N), wherein q, r, s, A, B, C, RA1, RA2, RB1, RB2, RC1, RC2, R3, R4, R5, R6, R14, R15, R16, and R17, are as defined herein, or a tautomer thereof, or a salt, particularly a pharmaceutically acceptable salt, thereof.揭示了具有化学式(I-N)的化合物,其中q、r、s、A、B、C、RA1、RA2、RB1、RB2、RC1、RC2、R3、R4、R5、R6、R14、R15、R16和R17如本文所定义,或其互变异构体,或其盐,特别是其药用可接受盐。
-
[EN] MODULATORS OF STIMULATOR OF INTERFERON GENES (STING) USEFUL IN TREATING HIV<br/>[FR] MODULATEURS DE STIMULATEUR DES GÈNES (STING) D'INTERFÉRON UTILES DANS LE TRAITEMENT DU VIH申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2019069269A1公开(公告)日:2019-04-11Disclosed are compounds having the formula: (I-N) wherein q, r, s, A, B, C, RA1, RA2, RB1, RB2, RC1, RC2, R3, R4, R5, R6, R14, R15, R16, and R17, are as defined herein, or a tautomer thereof, or a salt, particularly a pharmaceutically acceptable salt, thereof, along with combinations thereof, all of which are useful in HIV therapies.揭示了具有以下式的化合物:(I-N)其中q、r、s、A、B、C、RA1、RA2、RB1、RB2、RC1、RC2、R3、R4、R5、R6、R14、R15、R16和R17如本文所定义,或其互变异构体,或其盐,特别是其药用可接受盐,以及其组合物,所有这些在HIV疗法中是有用的。
-
[EN] PROCESSES USEFUL FOR THE SYNTHESIS OF (R)-1-{2-[4'-(3-METHOXYPROPANE-1-SULFONYL)-BIPHENYL-4-YL]-ETHYL}-2-METHYL-PYRROLIDINE<br/>[FR] PROCÉDÉS UTILES POUR LA SYNTHÈSE DE LA (R)-1-{2-[4'-(3-MÉTHOXYPROPANE-1-SULFONYL)-BIPHÉNYL-4-YL]-ÉTHYL}-2-MÉTHYL-PYRROLIDINE申请人:ARENA PHARM INC公开号:WO2009128907A1公开(公告)日:2009-10-22Processes useful for making a pharmaceutically useful compound according to Formula (I), forms of such a compound, and intermediates useful in such processes are described.根据公式(I)制备药用化合物的有用过程,以及该化合物的形式和在这些过程中有用的中间体被描述。
表征谱图
-
氢谱1HNMR
-
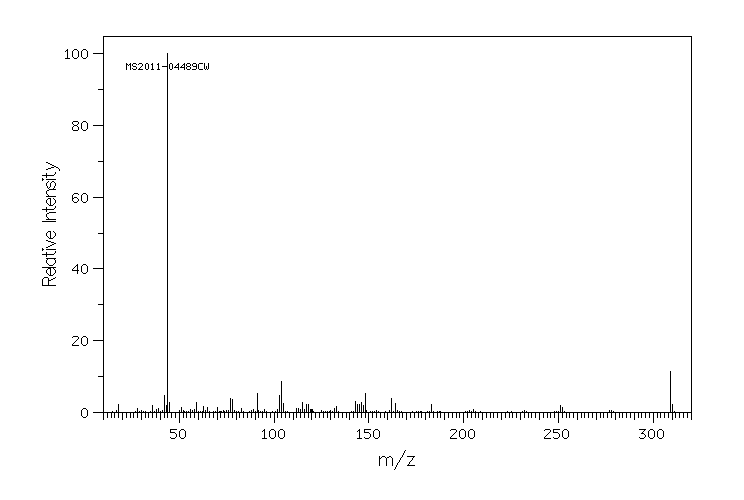
质谱MS
-
碳谱13CNMR
-
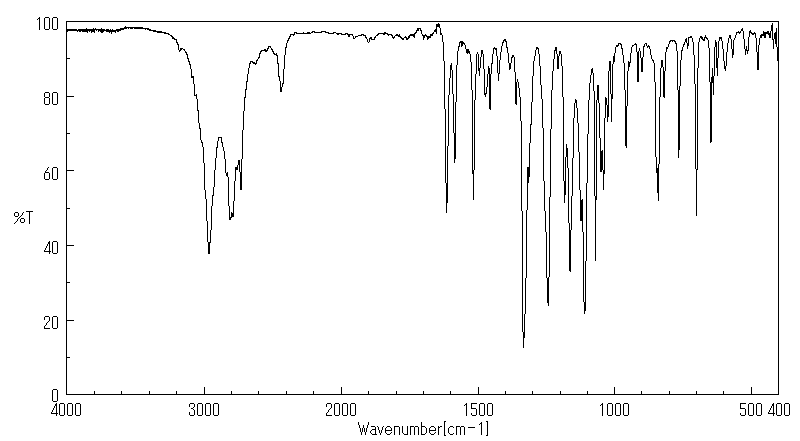
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息