3-甲硫基噻吩 | 20731-74-2
中文名称
3-甲硫基噻吩
中文别名
——
英文名称
3-(methylthio)thiophene
英文别名
3-(methylsulfanyl)thiophene;3-methylsulfanylthiophene
CAS
20731-74-2
化学式
C5H6S2
mdl
MFCD00068009
分子量
130.235
InChiKey
OTYBVBDWIKXFDO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:89-92°C 15mm
-
保留指数:1045;1048;1070;1054;1056;1059;1070;1076;1080;1060
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:7
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:53.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
海关编码:2934999090
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:存储条件:2-8℃,避光,干燥,密封。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-[(2-cyanoethyl)sulfanyl]thiophene 437711-55-2 C7H7NS2 169.271 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-(methylsulfinyl)thiophene 133361-97-4 C5H6OS2 146.234 —— 2-Bromo-4-(methylthio)thiophene 95191-79-0 C5H5BrS2 209.131 —— 2-methyl-4-(methylthio)thiophene 943867-44-5 C6H8S2 144.262 —— 2-bromo-3-(methylthio)thiophene 94781-39-2 C5H5BrS2 209.131 —— 2-iodo-3-(methylthio)thiophene 135528-12-0 C5H5IS2 256.131 —— 2-methyl-3-(methylthio)thiophene 159144-70-4 C6H8S2 144.262
反应信息
-
作为反应物:描述:3-甲硫基噻吩 在 N-溴代丁二酰亚胺(NBS) 、 正丁基锂 作用下, 以 溶剂黄146 为溶剂, 反应 3.25h, 生成 2-(1-Hydroxyethyl)-3-methylthiothiophene参考文献:名称:The directing ability of the methylthio substituent in lithiation reactions of thiophenes摘要:DOI:10.1021/jo00207a019
-
作为产物:参考文献:名称:有机硫化物的生物转化。第 12 部分。Helminthosporium sp.将杂环硫化物转化为手性亚砜。NRRL 4671 和伊莎贝拉被孢霉ATCC 42613摘要:已经使用真菌生物催化剂 Helminthosporium 物种 NRRL 4671 和 Mortierella isabellina ATCC 42613 对一系列杂环前手性硫化物对映选择性氧化为手性亚砜进行了研究。甲基硫代呋喃基和-噻吩底物在低至中等纯度的生物反式转化中产生 (S)-构型产物与 H. 物种,但氮原子位于距硫中心 8-10 A 最佳距离的吡啶基硫化物产生高对映体纯度的 (S) 亚砜。M. isabellina 对适当取代的苯并噻烷底物的生物转化也产生了高对映体纯度的产物,但在硫处具有 (R) 构型。底物的可接受性以及 H. 物种和 M. 对硫氧化的构型。DOI:10.1139/cjc-77-4-463
文献信息
-
Nickel-Catalyzed Reversible Functional Group Metathesis between Aryl Nitriles and Aryl Thioethers作者:Tristan Delcaillau、Philip Boehm、Bill MorandiDOI:10.1021/jacs.1c00529日期:2021.3.17We describe a new functional group metathesis between aryl nitriles and aryl thioethers. The catalytic system nickel/dcype is essential to achieve this fully reversible transformation in good to excellent yields. Furthermore, the cyanide- and thiol-free reaction shows high functional group tolerance and great efficiency for the late-stage derivatization of commercial molecules. Finally, synthetic applications
-
Nickel‐Catalyzed Inter‐ and Intramolecular Aryl Thioether Metathesis by Reversible Arylation作者:Tristan Delcaillau、Alessandro Bismuto、Zhong Lian、Bill MorandiDOI:10.1002/anie.201910436日期:2020.1.27A nickel-catalyzed aryl thioether metathesis has been developed to access high-value thioethers. 1,2-Bis(dicyclohexylphosphino)ethane (dcype) is essential to promote this highly functional-group-tolerant reaction. Furthermore, synthetically challenging macrocycles could be obtained in good yield in an unusual example of ring-closing metathesis that does not involve alkene bonds. In-depth organometallic
-
[EN] PYRROLE AND PYRAZOLE DERIVATIVES AS POTENTIATORS OF GLUTAMATE RECEPTORS<br/>[FR] COMPOSES DE PYRROLE ET PYRAZOLE PRESENTANT UN EFFET POTENTIATEUR SUR LES RECEPTEURS DU GLUTAMATE申请人:LILLY CO ELI公开号:WO2005040110A1公开(公告)日:2005-05-06The present invention relates to pyrrole and pyrazole compounds of formula (I) and their pharmaceutically acceptable salts, and further relates to their use in treating schizophrenia, cognitive deficits associated with schizophrenia, Alzheimer's disease, dementia of the Alzheimer's type, mild cognitive impairment, or depression. The compounds act as potentiators on glutamate receptors, in particular AMPA and the GluR family.
-
Metal-free synthesis of biaryls from aryl sulfoxides and sulfonanilides via sigmatropic rearrangement作者:Akira Yoshida、Koichi Okamoto、Tomoyuki Yanagi、Keisuke Nogi、Hideki YorimitsuDOI:10.1016/j.tet.2020.131232日期:2020.12dehydrative metal-free construction of the corresponding unsymmetrical biaryls. The reaction would proceed via (1) the activation of aryl sulfoxide with the anhydride, (2) interrupted Pummerer reaction of the resulting arylsulfonium with sulfonanilide, (3) [3,3] sigmatropic rearrangement to cleave the transient S–N bond and to form the prospective biaryl C–C bond, and (4) global aromatization. The
-
一种含氧族元素的并五稠环共轭分子与其衍 生物的合成方法以及用途
表征谱图
-
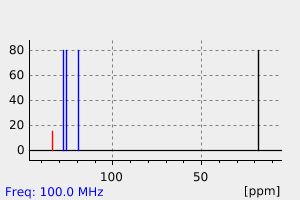
氢谱1HNMR
-
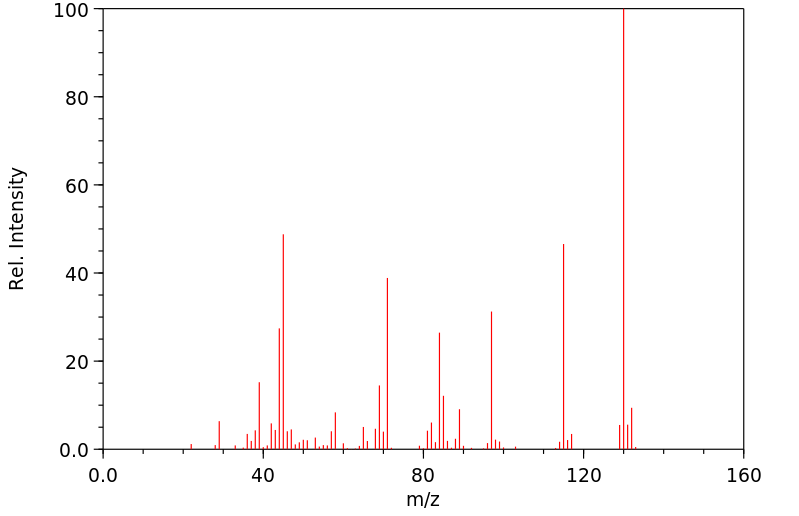
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯