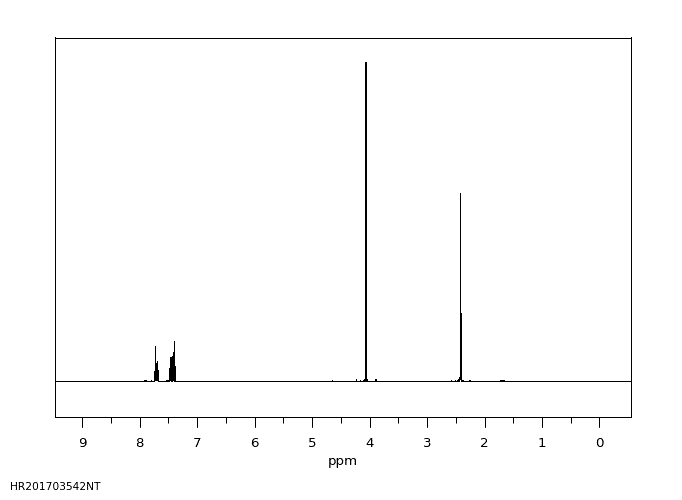3-甲苯酰基乙腈 | 53882-81-8
中文名称
3-甲苯酰基乙腈
中文别名
3-甲基苯甲酰乙腈;3-甲基-B-氧代苯丙腈
英文名称
(3-methylbenzoyl)acetonitrile
英文别名
3-oxo-3-(m-tolyl)propanenitrile;3-(3-Methylphenyl)-3-oxopropanenitrile
CAS
53882-81-8
化学式
C10H9NO
mdl
MFCD00067922
分子量
159.188
InChiKey
IVLKDYOTZMFMLO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:73 °C
-
沸点:318.6±25.0 °C(Predicted)
-
密度:1.081±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:40.9
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:6.1
-
危险品标志:Xn
-
危险类别码:R20/21/22,R36/37/38
-
危险品运输编号:3439
-
海关编码:2926909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S26,S36/37/39
-
危险性防范说明:P261,P264,P270,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P330,P362,P403+P233,P501
-
危险性描述:H302+H312+H332,H315,H319,H335
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
| Name: | 3-(3-Methylphenyl)-3-oxopropanenitrile 97% Material Safety Data Sheet |
| Synonym: | 3-Toluoylacetonitril |
| CAS: | 53882-81-8 |
Synonym:3-Toluoylacetonitril
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 53882-81-8 | 3-(3-Methylphenyl)-3-oxopropanenitrile | 97% | unlisted |
Risk Phrases: 20/21/22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. Harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
Harmful if inhaled. Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 53882-81-8: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: cream to tan
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 73 - 75 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H9NO
Molecular Weight: 159
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Reducing agents, strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen cyanide, nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 53882-81-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-(3-Methylphenyl)-3-oxopropanenitrile - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: NITRILES, SOLID, TOXIC, N.O.S.*
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
IMO
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
RID/ADR
Shipping Name: NITRILES, TOXIC, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
WGK (Water Danger/Protection)
CAS# 53882-81-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 53882-81-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 53882-81-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3'-甲基苯乙酮 1-(m-tolyl)ethanone 585-74-0 C9H10O 134.178 2-溴-1-间甲苯-乙酮 2-bromo-3'-methylacetophenone 51012-64-7 C9H9BrO 213.074 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-3-(dimethylamino)-2-(3-methylbenzoyl)prop-2-enenitrile 1319069-56-1 C13H14N2O 214.267 2,2-二氯-1-(3-甲基苯基)乙酮 2,2-dichloro-1-(m-tolyl)ethan-1-one 144660-10-6 C9H8Cl2O 203.068
反应信息
-
作为反应物:描述:3-甲苯酰基乙腈 在 水 、 sodium 、 铁粉 、 potassium carbonate 、 氯化铵 、 溶剂黄146 、 三乙胺 、 三氯氧磷 作用下, 以 四氢呋喃 、 乙醇 、 二氯甲烷 、 N,N-二甲基乙酰胺 、 水 为溶剂, 反应 114.0h, 生成 5-[2-amino-4-(trifluoromethyl)benzyl]-2-chloro-N-[(1R)-1-cyclobutylethyl]-6-(3-methylphenyl)-5H-pyrrolo[3,2-d]pyrimidin-4-amine参考文献:名称:[EN] SUBSTITUTED PYRROLOPYRIMIDINES AS HDM2 INHIBITORS
[FR] PYRROLOPYRIMIDINES SUBSTITUÉES EN TANT QU'INHIBITEURS DE HDM2摘要:公开号:WO2014100071A3 -
作为产物:参考文献:名称:双级抗疟唑并[3,4-b]吡啶的合成及其构效关系。摘要:疟疾仍然是最致命的传染病之一,每年导致成千上万的死亡,主要是在幼儿和孕妇中。在这里,我们报道了针对恶性疟原虫(疟疾最致命的物种)的一系列吡唑并[3,4- b ]吡啶的发现和衍生化。该系列中的命中化合物在体外显示出亚微摩尔对寄生虫的红细胞内阶段具有高活性,对人成纤维细胞BJ和肝HepG2细胞系几乎没有毒性。此外,我们的命中化合物对寄生虫的肝期表现出良好的活性,但对配子体阶段的活性却很小。包括杀死率,对接率和分子动力学研究在内的寄生虫学资料表明,我们的化合物可能靶向细胞色素bc 1的Q o结合位点。DOI:10.1021/acs.jmedchem.0c01152
文献信息
-
[EN] PIKFYVE KINASE INHIBITORS<br/>[FR] INHIBITEURS DE KINASE PIKFYVE申请人:ACURASTEM INCORPORATED公开号:WO2021163727A1公开(公告)日:2021-08-19The present invention relates to compounds useful as inhibitors of phosphatidylinositol-3-phosphate 5-kinase (PIKfyve) as well as their use for treating diseases and disorders associated with PIKfyve.
-
Palladium-Catalyzed Carbonylative α-Arylation of <i>tert</i>-Butyl Cyanoacetate with (Hetero)aryl Bromides作者:Mikkel T. Jensen、Martin Juhl、Dennis U. Nielsen、Mikkel F. Jacobsen、Anders T. Lindhardt、Troels SkrydstrupDOI:10.1021/acs.joc.5b02897日期:2016.2.19A three-component coupling protocol has been developed for the generation of 3-oxo-3-(hetero)arylpropanenitriles via a carbonylative palladium-catalyzed α-arylation of tert-butyl 2-cyanoacetates with (hetero)aryl bromides followed by an acid-mediated decarboxylation step. Through the combination of only a stoichiometric loading of carbon monoxide and mild basic reaction conditions such as MgCl2 and
-
Chlorophosphines as auxiliary ligands in ruthenium-catalyzed nitrile hydration reactions: application to the preparation of β-ketoamides作者:Rebeca González-Fernández、Pedro J. González-Liste、Javier Borge、Pascale Crochet、Victorio CadiernoDOI:10.1039/c5cy02142a日期:——heteroaryl or alkyl group). In the reaction medium, the coordinated chlorophosphines readily undergo hydrolysis to generate the corresponding phosphinous acids PR2OH, which are well-known “cooperative” ligands for this catalytic transformation. Among the complexes employed, best results were obtained with [RuCl2(η6-p-cymene)P(4-C6H4F)2Cl}]. Performing the catalytic reactions at 40 °C with 2 mol% of this腈成酰胺,在中性条件下的水的催化水合,已使用一系列芳烃-钌(的研究II含有市售chlorophosphines作为辅助配位体)配合物,即化合物将[RuCl 2(η 6 - p(-cymene) PR 2 Cl)](R =芳基,杂芳基或烷基)。在反应介质中,配位的氯膦易于水解生成相应的次膦酸PR 2 OH,次膦酸PR 2 OH是该催化转化的众所周知的“配合”配体。中所采用的复合物,用将[RuCl获得最好的结果2(η 6 - p-cymene)P(4-C 6 H 4 F)2 Cl}]。在40°C下用2 mol%的这种络合物进行催化反应,可以将各种有机腈以高收率和较短时间选择性地转化为相应的伯酰胺。的应用将[RuCl 2(η 6 - p -cymene)P(4-C 6 H ^ 4 F)2氯}]在合成有用β酮酰胺的制备也提出。
-
Visible Light Promotes Decyanation Esterification Reaction of β-Ketonitriles with Dioxygen and Alcohols to α-Ketoesters作者:Cheng Guo、Chen Xu、Nan-Nan Zhang、Xiao-ji Li、Yan-qin Ge、Pin-hui DiaoDOI:10.1055/s-0036-1591943日期:2018.5A green and mild method has been developed for the conversion of β-ketonitriles into α-ketoesters under catalyst-free conditions. A plausible mechanism is that visible light promotes singlet oxygen generation to form the products through oxidative C–H bond functionalization and C–C σ -bond cleavage.开发了一种绿色温和的方法,用于在无催化剂条件下将 β-酮腈转化为 α-酮酯。一个合理的机制是可见光通过氧化 C-H 键功能化和 C-C σ 键裂解促进单线态氧的产生以形成产物。
-
Facile photochemical synthesis of α-ketoamides and quinoxalines from amines and benzoylacetonitrile under mild conditions作者:Chao Zhou、Pinhui Diao、Xiaoji Li、Yanqin Ge、Cheng GuoDOI:10.1016/j.cclet.2018.06.019日期:2019.2Abstract A selective protocol for the synthesis of either α -ketoamides or quinoxaline derivatives under the same reaction conditions has been achieved simply by varying substitution number of amino-groups. The method features metal-free, room temperature and broad substrate scopes as well as no extra oxidant. This process applies to various substituent groups and gives products in moderate to good
表征谱图
-
氢谱1HNMR
-
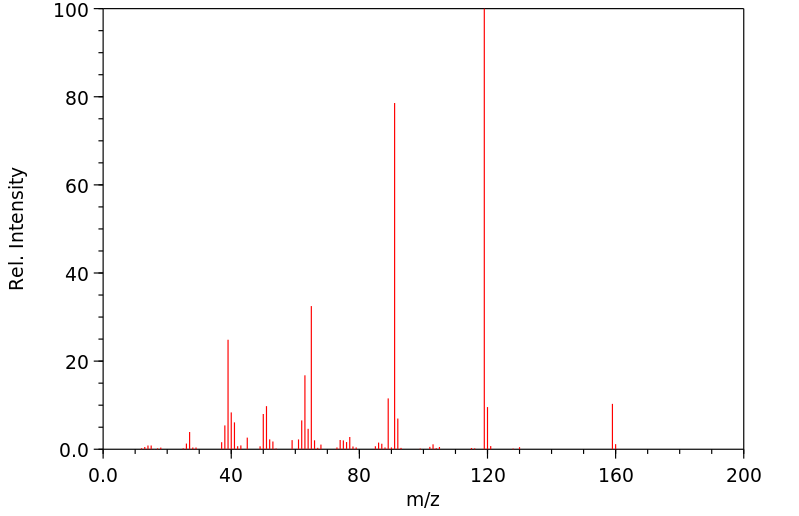
质谱MS
-
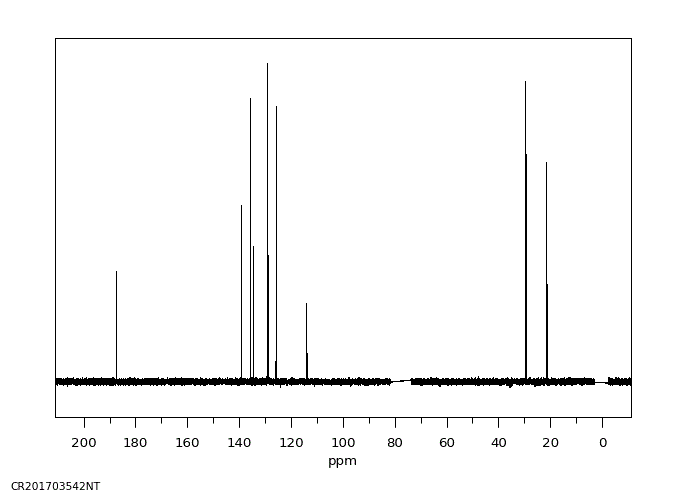
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷