3-硝基苯甲醚 | 555-03-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:36-38°C
-
沸点:258°C
-
密度:1.373
-
闪点:>110°C
-
溶解度:酒精:可溶(lit.)
-
保留指数:1273
-
稳定性/保质期:
-
稳定性[10]:稳定。
-
禁配物[11]:强氧化剂、强还原剂、强酸和强碱。
-
应避免的条件[12]:受热。
-
聚合危害[13]:不发生聚合。
-
分解产物[14]:氮氧化物。
-
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R22
-
RTECS号:BZ8787000
-
海关编码:29093090
-
包装等级:III
-
危险类别:6.1(b)
-
WGK Germany:3
-
危险品运输编号:3458
-
储存条件:1. **储存注意事项**:应将物品储存在阴凉、通风良好的库房中,远离火源和热源,并确保包装密封。切勿与氧化剂、还原剂、酸类、碱类以及食用化学品混放,避免混合储存。配备相应的消防设备,并确保储区备有合适的材料以处理可能的泄漏。 2. **包装方法**:对于液体产品,使用小开口钢桶;螺纹口玻璃瓶、铁盖压口玻璃瓶、塑料瓶或金属桶(罐),并外加普通木箱。对于固体产品,则应采用塑料袋或双层牛皮纸袋外加全开口或中开口钢桶;螺纹口玻璃瓶、铁盖压口玻璃瓶、塑料瓶或金属桶(罐)同样需外加普通木箱,或者选择螺纹口玻璃瓶、塑料瓶或镀锡薄钢板桶(罐),并外加满底板花格箱、纤维板箱或胶合板箱。
制备方法与用途
用途:用于有机合成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 间硝基苯酚 meta-nitrophenol 554-84-7 C6H5NO3 139.111 2-甲氧基-4-硝基苯胺 2-methoxy-4-nitrophenylamine 97-52-9 C7H8N2O3 168.152 2-硝基-4-甲氧基苯胺 4-methoxy-2-nitroaniline 96-96-8 C7H8N2O3 168.152 —— methyl 3-nitrophenyl carbonate 17175-17-6 C8H7NO5 197.147 1-甲氧基-3-亚硝基苯 1-methoxy-3-nitrosobenzene 26595-63-1 C7H7NO2 137.138 4-溴-3-硝基苯甲醚 4-bromo-3-nitroanizole 5344-78-5 C7H6BrNO3 232.034 间氨基苯甲醚 m-Anisidine 536-90-3 C7H9NO 123.155 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 间硝基苯酚 meta-nitrophenol 554-84-7 C6H5NO3 139.111 4-硝基愈创木酚 2-methoxy-4-nitrophenol 3251-56-7 C7H7NO4 169.137 2-甲氧基-4-硝基苯胺 2-methoxy-4-nitrophenylamine 97-52-9 C7H8N2O3 168.152 —— 3-nitrophenyl acetate 1523-06-4 C8H7NO4 181.148 2-硝基-4-甲氧基苯胺 4-methoxy-2-nitroaniline 96-96-8 C7H8N2O3 168.152 1-甲氧基-3-亚硝基苯 1-methoxy-3-nitrosobenzene 26595-63-1 C7H7NO2 137.138 N-(3-甲氧基苯基)羟胺 N-(3-methoxyphenyl)hydroxylamine 24171-80-0 C7H9NO2 139.154 4-氯-3-硝基苯甲醚 2-chloro-5-methoxynitrobenzene 10298-80-3 C7H6ClNO3 187.583 6-甲氧基-2-硝基苯酚 6-methoxy-2-nitrophenol 15969-08-1 C7H7NO4 169.137 4-碘基-3-硝基苯甲醚 1-iodo-4-methoxy-2-nitrobenzene 58755-70-7 C7H6INO3 279.034 4-溴-3-硝基苯甲醚 4-bromo-3-nitroanizole 5344-78-5 C7H6BrNO3 232.034 2-甲氧基-6-硝基苯胺 2-methoxy-6-nitroaniline 16554-45-3 C7H8N2O3 168.152 2-甲氧基-1,4-二硝基-苯 2,5-dinitro-anisole 3962-77-4 C7H6N2O5 198.135 1,2-二硝基-4-甲氧基苯 3,4-dinitroanisole 4280-28-8 C7H6N2O5 198.135 2-氯甲基-5-硝基茴香醚 2-chloromethyl-5-nitro-anisole 101080-01-7 C8H8ClNO3 201.609 —— 2-methoxy-4-nitro-N-phenylaniline 82040-92-4 C13H12N2O3 244.25 (2-甲氧基-4-硝基苯基)乙腈 (2-methoxy-4-nitrophenyl)acetonitrile 313233-24-8 C9H8N2O3 192.174 —— 1-(chloromethyl)-4-methoxy-2-nitrobenzene 101080-02-8 C8H8ClNO3 201.609 —— 2-methoxy-N-(4-methoxyphenyl)-4-nitroaniline —— C14H14N2O4 274.276 间氨基苯甲醚 m-Anisidine 536-90-3 C7H9NO 123.155 (4-甲氧基-2-硝基苯基)乙腈 2-(4-methoxy-2-nitrophenyl)acetonitrile 105003-90-5 C9H8N2O3 192.174 1-甲氧基-2,3-二硝基苯 2,3-dinitroanisole 16315-07-4 C7H6N2O5 198.135 2-甲氧基-4-硝基苯甲酸 4-nitro-o-anisic acid 2597-56-0 C8H7NO5 197.147 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:金催化硝基芳烃的直接加氢偶联以合成芳族偶氮化合物摘要:偶氮键是一种突出的化学基序,已在材料科学,制药和农用化学品中得到了广泛的应用。本文描述了偶氮芳烃的一种可持续的异质金催化合成方法。通过使用分子H 2形成N HN键,对可用的硝基芳烃进行脱氧和选择性连接没有任何外部添加剂。由于金属和载体之间具有独特而显着的协同作用,因此亚硝基和羟胺中间体之间可以轻松进行表面介导的缩合,并在温和的反应条件下以高度选择性的方式进行所需的转化。该方案可耐受多种官能团,并为环保合成对称或不对称芳族偶氮化合物提供了一种通用的通用方法。DOI:10.1002/anie.201404543
-
作为产物:描述:参考文献:名称:Reactions of aryl diazonium salts and arylazo alkyl ethers. 7. Kinetic studies of the decomposition of Z ethers derived from some substituted 2-nitrobenzenediazonium salts摘要:DOI:10.1021/jo00140a017
-
作为试剂:参考文献:名称:310.喹啉系列中形成无水碱的一个例子摘要:DOI:10.1039/jr9340001419
文献信息
-
Further Evidence for the Triplet Mechanism in the Photosubstitution of Nitroaryl Ethers in Alkaline Medium.作者:João Baptista Sargi Bonilha、Antonio Claudio Tedesco、Lazaro Cicero Nogueira、Maria Teresa Ribeiro Silva Diamantino、Júlio Cesar CarreiroDOI:10.1016/s0040-4020(01)89887-2日期:1993.4Mechanistic studies show that nitroaryl ethers (3-nitroanisole, 3-nitrophenetole, n-butyl 3-nitrophenyl ether, 2-chloro-5-nitroanisole, 2-bromo-5-nitroanisole and 3,5-dinitro anisole) undergo nucleophilic aromatic photosubstitution with hydroxide ions through an SN23Ar* mechanism. An investigation of the quenching of exited states of nitroanyl ethers by bromide and thiosulfate ions in aqueous solutions
-
Microwave-Assisted Rapid and efficient Reduction of Aromatic Nitro Compounds to Amines with Propan-2-ol over Nanosized Perovskite-type SmFeO<sub>3</sub> powder as a New Recyclable Heterogeneous Catalyst作者:Saeid Farhadi、Firouzeh Siadatnasab、Maryam KazemDOI:10.3184/174751911x12964930076647日期:2011.2
Nanosized perovskite-type SmFeO3 powder, prepared through the thermal decomposition of Sm[Fe(CN)6].4H2O with an average particle diameter of 28 nm and a specific surface area of 42 m2 g−1, was used as a recyclable heterogeneous catalyst for the efficient and selective reduction of aromatic nitro compounds into the corresponding amines by using propan-2-ol as a hydrogen donor (reducing agent) and KOH as a promoter under microwave irradiation. This highly regio- and chemoselective catalytic method is fast, clean, inexpensive, high yielding and also compatible with the substrates containing easily reducible functional groups. In addition, the nanosized SmFeO3 catalyst can be reused without loss of activity.
-
Enhanced catalytic performance of cobalt nanoparticles coated with a N,P-codoped carbon shell derived from biomass for transfer hydrogenation of functionalized nitroarenes作者:Yanan Duan、Tao Song、Xiaosu Dong、Yong YangDOI:10.1039/c8gc00619a日期:——(NPs) coated with a N,P-codoped carbon shell derived from naturally renewable biomass and earth-abundant, low-cost cobalt salt and PPh3. The entire process is operationally simple, straightforward, cost-effective and environmentally benign and can be used in mass production for practical application. The resultant catalysts allow for highly efficient and selective transfer hydrogenation of functionalized开发用于有机转化的大量可利用的贱金属催化剂仍然是化学研究的重要目标。在本文中,我们报道了第一种简便,快速,活性,廉价且可重复使用的钴纳米颗粒(NPs)的制备方法,该纳米颗粒涂有N,P掺杂的碳壳,该壳由天然可再生生物质和地球上丰富的低成本钴盐和PPh 3制成。。整个过程操作简单,直接,具有成本效益且对环境无害,可用于实际生产中的批量生产。所得的催化剂允许使用甲酸或甲酸铵作为氢供体将官能化的硝基芳烃高效且选择性地转移氢化成相应的苯胺。均匀掺入碳晶格中的N和P与包封的Co NPs表现出协同效应,以工程化催化剂的结构和组成,从而大大提高了催化效率。最具活性的催化剂Co @ NPC-800表现出出色的活性和选择性,可将官能化的硝基芳烃还原为苯胺,特别是装饰有易于还原的官能团的苯胺。
-
Reduction of Nitroarenes to Aromatic Amines with Nanosized Activated Metallic Iron Powder in Water作者:Lei Wang、Pinhua Li、Zongtao Wu、Jincan Yan、Min Wang、Yanbin DingDOI:10.1055/s-2003-41021日期:——A practical reduction of nitroarenes with nanosized activated metallic iron powder in water at 210 °C (near-critical water) has been developed. The reduction generates the corresponding aromatic amines in excellent yields.
-
Stable and reusable platinum nanocatalyst: an efficient chemoselective reduction of nitroarenes in water作者:Surya Srinivas Kotha、Nidhi Sharma、Govindasamy SekarDOI:10.1016/j.tetlet.2016.01.111日期:2016.3Binaphthyl stabilized Pt nanoparticles (Pt-BNP) have been synthesized, characterized, and utilized as an efficient heterogeneous catalyst for chemoselective reduction of nitroarenes at room temperature in water. Several sensitive functional groups like ketone, ester, acid, amide, halides, and nitrile were well tolerated in this chemoselective reduction. The Pt-BNP catalyst was quantitatively recovered
表征谱图
-
氢谱1HNMR
-
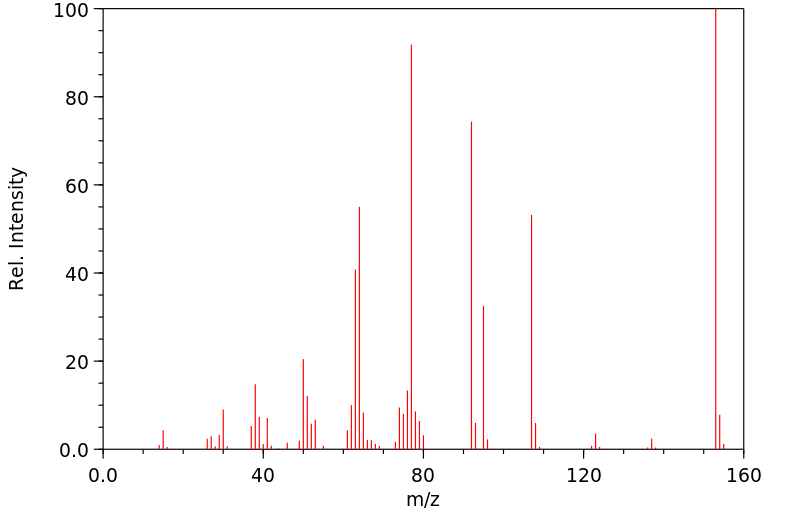
质谱MS
-
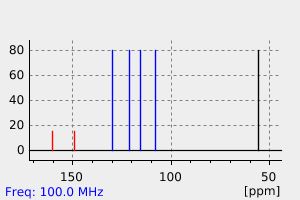
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息