4,6-癸二炔 | 16387-71-6
中文名称
4,6-癸二炔
中文别名
——
英文名称
deca-4,6-diyne
英文别名
4,6-decadiyne
CAS
16387-71-6
化学式
C10H14
mdl
MFCD00041645
分子量
134.221
InChiKey
LIWZSNTUMSGWTF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-6.35°C (estimate)
-
沸点:76°C 2mm
-
密度:0,869 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
海关编码:2901299090
-
储存条件:2-8℃
SDS
4,6-癸二炔 修改号码:2
模块 1. 化学品
产品名称: 4,6-Decadiyne
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 易燃液体和蒸气
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,6-癸二炔
百分比: >97.0%(GC)
CAS编码: 16387-71-6
分子式: C10H14
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
4,6-癸二炔 修改号码:2
模块 4. 急救措施
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于冷藏防爆冰箱。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 浅黄色-红黄色
气味: 无资料
pH: 无数据资料
4,6-癸二炔 修改号码:2
模块 9. 理化特性
熔点: 无资料
沸点/沸程 96 °C/2kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.82
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
避免接触的条件: 热敏, 气敏
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: III
4,6-癸二炔 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4,6-Decadiyne
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第3级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 易燃液体和蒸气
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,6-癸二炔
百分比: >97.0%(GC)
CAS编码: 16387-71-6
分子式: C10H14
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
4,6-癸二炔 修改号码:2
模块 4. 急救措施
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于冷藏防爆冰箱。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 浅黄色-红黄色
气味: 无资料
pH: 无数据资料
4,6-癸二炔 修改号码:2
模块 9. 理化特性
熔点: 无资料
沸点/沸程 96 °C/2kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.82
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
反应性: 未报道特殊反应性。
避免接触的条件: 热敏, 气敏
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: III
4,6-癸二炔 修改号码:2
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:4,6-癸二炔 在 mercury dichloride 作用下, 生成 4,6-decanedione参考文献:名称:Grignard; Tcheoufaki, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1929, vol. 188, p. 529摘要:DOI:
-
作为产物:描述:1-戊炔 在 copper(l) iodide 、 4,7-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-1,10-phenanthroline 、 氧气 作用下, 以 水 为溶剂, 80.0 ℃ 、300.01 kPa 条件下, 反应 6.0h, 以75%的产率得到4,6-癸二炔参考文献:名称:金属硅化物催化水中好氧串联去甲硅烷基化/玻璃化反应摘要:合成了PEG接枝的氮配体。相应的铜配合物可作为金属硅纳米反应器,用于水中TMS保护的炔烃的好氧串联去甲硅烷基化/玻璃偶联。该协议也适用于末端炔烃的无碱均偶联。金属硅油催化剂可循环使用5次,反应性损失很小。DOI:10.1039/c8gc03815e
文献信息
-
Visual and tear function improvement after superficial phototherapeutic keratectomy (PTK) for mid-stromal corneal scarring作者:Murat Dogru、Chikako Katakami、Masato Miyashita、Etsuko Hida、Mamoru Uenishi、Kazuaki Tetsumoto、Sanae Kanno、Teruo Nishida、Akio YamanakaDOI:10.1038/eye.2000.204日期:2000.9Purpose To study the changes in visual and tear film function following superficial excimer laser phototherapeutic keratectomy in patients with mid-stromal corneal scars. Methods Fourteen eyes of 14 patients with mid-stromal corneal scars seen at the Department of Ophthalmology at Kobe Kaisei Hospital underwent superficial phototherapeutic keratectomy (PTK). The subjects underwent routine ophthalmic examinations, corneal sensitivity measurements, tear film break-up time (BUT), Schirmer test and tear film lipid layer interferometry. Thirty eyes of 15 normal control subjects were also studied. The patients and the control subjects were compared for pre-PTK tear function parameters and tear film lipid layer interferometry grade. The alterations in these parameters within 6 months following PTK were also determined. Results Visual improvement was achieved in 12 eyes (86%). A hyperopic shift was observed in all eyes. The average pre-PTK corneal sensitivity and tear film BUT were lower in patients compared with control subjects before PTK. Tear film lipid layer interferometry grades were also higher in the patients than the controls before PTK. All these parameters improved gradually and significantly after PTK. Schirmer test results did not show any significant alterations after PTK. Conclusion We conclude that PTK is an effective means of treating corneal scars and attaining visual improvement, even in cases with deeper corneal involvement, and may obviate the need for corneal transplantation. Simultaneous improvements in corneal sensitivity and tear film stability suggest favourable effects of PTK on the ocular surface.目的:研究浅层准分子激光角膜切削术(PTK)后,中层基质性角膜瘢痕患者视觉和泪膜功能变化。方法:在神户海星医院眼科,14名中层基质性角膜瘢痕患者接受浅层PTK手术,共14眼。术前进行常规眼科检查,测量角膜敏感性、泪膜破裂时间(BUT)、泪液分泌测试和泪膜脂质层干涉测量。还研究了15例正常对照者的共30眼。比较术前患者和对照者的泪膜功能参数和泪膜脂质层干涉测量分级。术后6个月内这些参数的变化也进行了研究。结果:12眼(86%)改善了视力。所有眼出现远视漂移。术前患者角膜敏感性和泪膜BUT低于对照组。术前患者的泪膜脂层干涉测量分级高于对照组眼。这些参数术后逐渐显著提高。PTK后Schaumberg试验结果没有显著变化。结论:,结论:我们认为PTK是治疗角膜瘢痕并获得视力提高的有效方法,即便是伴有更深层角膜病变者,并且可能不再需要进行角膜移植。角膜敏感性和泪膜稳定性同时提高,表明PTK对眼表有良好的效应。
-
Tailored Palladium Catalysts for Selective Synthesis of Conjugated Enynes by Monocarbonylation of 1,3‐Diynes作者:Jiawang Liu、Ji Yang、Carolin Schneider、Robert Franke、Ralf Jackstell、Matthias BellerDOI:10.1002/anie.201915386日期:2020.6.21'-binaphthalene (Neolephos), which permits the palladium-catalyzed selective carbonylation under mild conditions, providing a general preparation of functionalized 1,3-enynes in good-to-high yields with excellent chemoselectivities. Synthetic applications that showcase the possibilities of this novel methodology include an efficient one-pot synthesis of 4-aryl-4H-pyrans as well as the rapid construction of various
-
A one-pot synthesis of 1-arylalka-1,3-diynes by sequential acetylene zipper and Sonogashira reactions作者:Irina A. Balova、Svetlana N. Morozkina、David W. Knight、Sergei F. VasilevskyDOI:10.1016/s0040-4039(02)02496-6日期:2003.11,3-Diynes, formed in situ by base-induced acetylene zipper reactions, following anion quenching with water, undergo smooth Sonogashira-type couplings with functionalized aryl iodides, to give good overall yields of 1-arylalka-1,3-diynes.
-
Copper(<scp>i</scp>) chloride catalysed room temperature C<sub>sp</sub>–C<sub>sp</sub> homocoupling of terminal alkynes mediated by visible light作者:Arunachalam Sagadevan、Vaibhav Pramod Charpe、Kuo Chu HwangDOI:10.1039/c6cy01400c日期:——We developed a technique mediated by visible light for the aerobic homocoupling of terminal alkynes to synthesize 1,3-conjugated diynes using a copper(I) chloride catalyst at room temperature. Compared with previously reported thermal processes, this photochemical method is simple, uses only mild reaction conditions, produces high yields and works well for substrates with electron-withdrawing groups
-
Aldehyde Addition to 1,3-Butadiyne-Derived Zirconacyclocumulenes: Stereoselective Synthesis of <i>cis</i>-[3]Cumulenols作者:Xiaoping Fu、Yuanhong Liu、Yuxue LiDOI:10.1021/om100287d日期:2010.7.121,3-butadiynes is achieved cleanly under controlled reaction conditions, which provids a highly stereoselective method for the synthesis of tetra-substituted [3]cumulenols. DFT calculations suggest that there is an equilibrium between seven-membered zirconacyclocumulene (3) and the less stable but more reactive five-membered α-alkynylzirconaindene (2) in the process. The aldehyde reacts with the five-membered
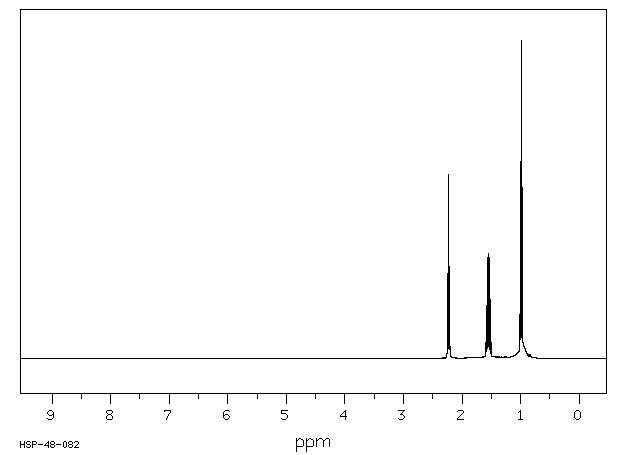
表征谱图
-
氢谱1HNMR
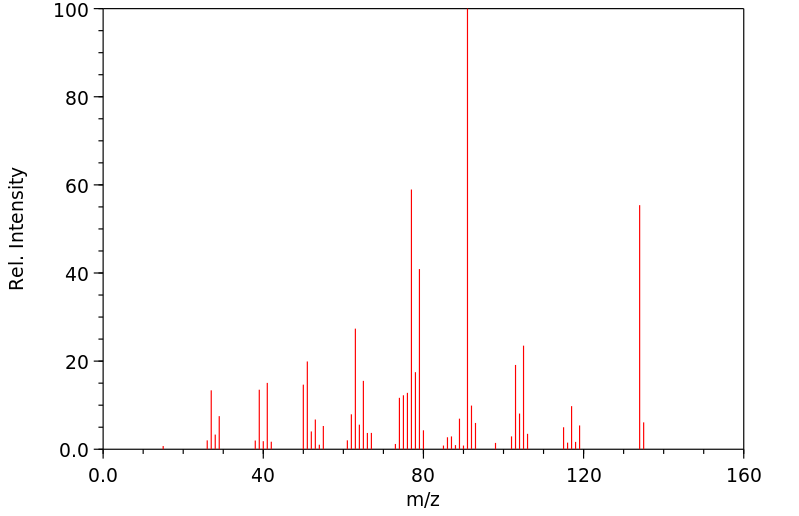
-
质谱MS
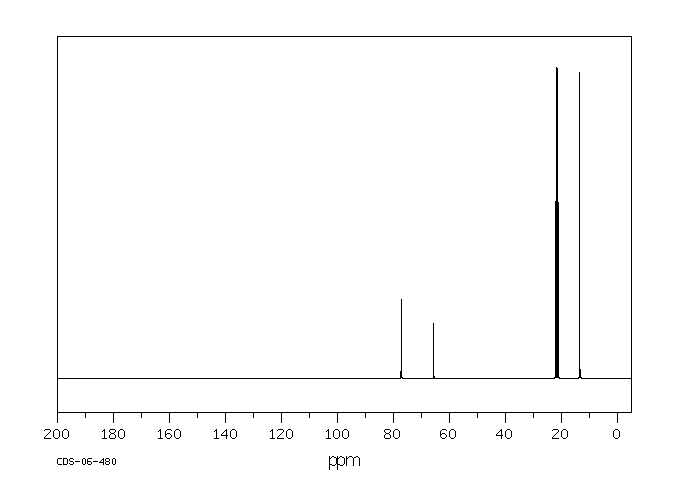
-
碳谱13CNMR
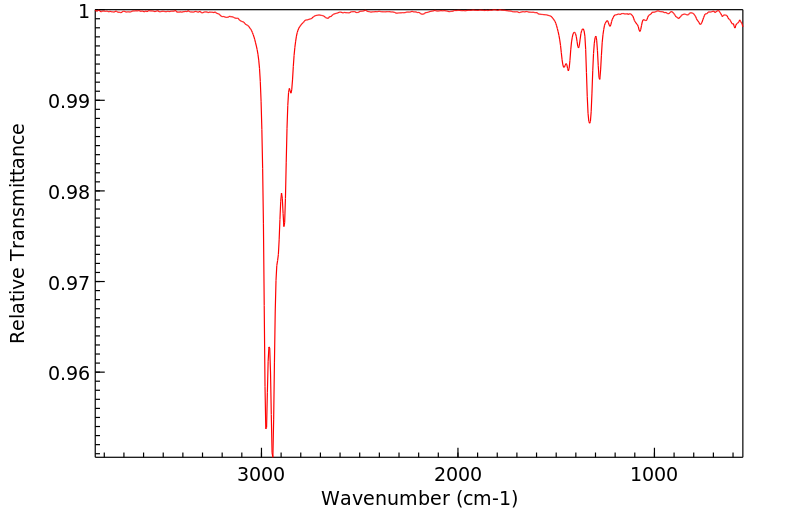
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-