4-乙基苄氯 | 1467-05-6
中文名称
4-乙基苄氯
中文别名
4-乙基氯苄;1-氯甲基-4-乙基苯
英文名称
4-ethylbenzylchloride
英文别名
1-(chloromethyl)-4-ethylbenzene;4-Ethylbenzyl chloride
CAS
1467-05-6
化学式
C9H11Cl
mdl
MFCD00039357
分子量
154.639
InChiKey
DUBCVXSYZVTCOC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-21°C
-
沸点:111°C/26mm
-
密度:1,05 g/cm3
-
闪点:111°C/26mm
-
保留指数:1184
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):3.1
-
重原子数:10
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:Xi
-
危险类别码:R34
-
危险品运输编号:1760
-
海关编码:2903999090
-
包装等级:III
-
危险类别:8
-
安全说明:S26,S36/37/39
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥的地方。确保工作间有足够的良好通风或排气装置。
SDS
Section I.Chemical Product and Company Identification
Chemical Name 4-Ethylbenzyl Chloride
(contains ca 30% 2-form)
Portland OR
Synonym Benzene, 1-(chloromethyl)-4-ethyl-(9 CI)
Chemical Formula C2H5C6H4CH2Cl
CAS Number 1467-05-6
Section II. Composition and Information on Ingredients
Toxicology Data
Chemical Name CAS Number Percent (%) TLV/PEL
4-Ethylbenzyl Chloride 1467-05-6 Min. 65.0(GC) Not available. Not available.
(contains ca 30% 2-form)
Section III. Hazards Identification
Acute Health Effects Corrosive to skin, eyes, and respiratory system. Liquid or spray mist may produce tissue damage, particularly in mucous
membranes of the eyes, mouth and respiratory tract. Skin contact may produce burns. Eye contact can result in corneal
damage or blindness. Inhalation of the spray mist may produce severe irritation of respiratory tract, characterized by
coughing, choking, or shortness of breath. Corrosive materials may cause serious injury if ingested.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Chronic Health Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged contact with spray mist may produce chronic eye irritation and severe skin irritation. Repeated or
prolonged exposure to spray mist may produce respiratory tract irritation leading to frequent attacks of bronchial
infection.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion DO NOT INDUCE VOMITING. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing,
perform mouth-to-mouth resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a
possible indication that the toxic material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Auto-Ignition Not available.
Flammability May be combustible at high temperature.
Flash Points Flammable Limits Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), halogenated compounds.
WARNING: Highly toxic HCl gas is produced during combustion.
Fire Hazards
Not available.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
4-Ethylbenzyl Chloride
(contains ca 30% 2-form)
Section VI. Accidental Release Measures
Spill Cleanup
Corrosive liquid.
Stop leak if without risk. Absorb with DRY earth, sand or other non-combustible material. DO NOT get water inside
Instructions
container. DO NOT touch spilled material. Use water spray curtain to divert vapor drift. Prevent entry into sewers,
basements or confined areas; dike if needed. Eliminate all sources of ignition. Consult federal, state, and/or local
authorities for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage CORROSIVE. Keep container dry. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the
container and store in a dry, cool place. Avoid excessive heat and light. Do not breathe gas/fumes/ vapor/spray. Never
Information
add water to this product. Wear suitable protective clothing. If you feel unwell, seek medical attention and show the label
when possible. Treat symptomatically and supportively.
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Face shield. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
specialist BEFORE handling this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Liquid. (Clear, colorless.) Not available.
1.05 (water=1)
Specific Gravity
Molecular Weight 154.64 Partition Coefficient Not available.
Boiling Point
Not available. Vapor Pressure Not available.
Melting Point Not available. Vapor Density Not available.
Refractive Index Not available. Volatility Not available.
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Eye Contact. Ingestion. Inhalation. Skin contact.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged contact with spray mist may produce chronic eye irritation and severe skin irritation. Repeated or
prolonged exposure to spray mist may produce respiratory tract irritation leading to frequent attacks of bronchial infection.
Acute Toxic Effects Corrosive to skin, eyes, and respiratory system. Liquid or spray mist may produce tissue damage, particularly in mucous
membranes of the eyes, mouth and respiratory tract. Skin contact may produce burns. Eye contact can result in corneal
damage or blindness. Inhalation of the spray mist may produce severe irritation of respiratory tract, characterized by
coughing, choking, or shortness of breath. Corrosive materials may cause serious injury if ingested.
Follow safe industrial hygiene practices and always wear proper protective equipment when handling this compound.
Continued on Next Page
4-Ethylbenzyl Chloride
(contains ca 30% 2-form)
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Class 8: Corrosive material.
PIN Number
Proper Shipping Name
Corrosive liquids, n.o.s.
Packing Group (PG) III
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This compound is ON the EPA Toxic Substances Control Act (TSCA) inventory list.
(EPA)
WHMIS Classification
CLASS E: Corrosive liquid.
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements R34- Causes burns.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-乙基甲苯 4-methylethylbenzene 622-96-8 C9H12 120.194 乙基苯 ethylbenzene 100-41-4 C8H10 106.167 4-乙基苯甲醛 4-ethylbenzylaldehyde 4748-78-1 C9H10O 134.178 4-氯甲基苯乙烯 4-Vinylbenzyl chloride 1592-20-7 C9H9Cl 152.623 4-乙基苄醇 4-ethylbenzyl alcohol 768-59-2 C9H12O 136.194 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-乙基甲苯 4-methylethylbenzene 622-96-8 C9H12 120.194 1-(4-氯甲基苯基)乙醇 1-(4-chloromethylphenyl)ethanol 76297-21-7 C9H11ClO 170.639 4-乙基苯基乙腈 (4-ethyl-phenyl)-acetonitrile 51632-28-1 C10H11N 145.204 4-乙基苯甲醛 4-ethylbenzylaldehyde 4748-78-1 C9H10O 134.178 4-氯甲基苯乙烯 4-Vinylbenzyl chloride 1592-20-7 C9H9Cl 152.623 4-乙基苄醇 4-ethylbenzyl alcohol 768-59-2 C9H12O 136.194 1-(溴甲基)-4-乙基苯 4-ethylbenzyl bromide 57825-30-6 C9H11Br 199.09 3-(4-乙基苯基)-1-丙醇 3-(4-ethylphenyl)propan-1-ol 180274-13-9 C11H16O 164.247 —— 1-(but-3-en-1-yl)-4-ethylbenzene —— C12H16 160.259
反应信息
-
作为反应物:描述:参考文献:名称:Rinkes, Recueil des Travaux Chimiques des Pays-Bas, 1945, vol. 64, p. 205,212摘要:DOI:
-
作为产物:参考文献:名称:Fischer, Alfred; Henderson, George N., Canadian Journal of Chemistry, 1981, vol. 59, p. 2314 - 2327摘要:DOI:
-
作为试剂:描述:参考文献:名称:The Analogs of 8-D-Homoarginin-vasopressin with O-Substituted Phenylalanine in Position 2: Synthesis and Some Biological Properties摘要:在p-甲基苄基胺树脂上使用固相方法合成了四个非编码氨基酸类似加压素的类似物,其中包括D-同型精氨酸(位于第8位)和o-取代的L-或D-苯丙氨酸(位于第2位)。[L-Phe(o-Me)2,D-Har8]加压素(I),[D-Phe(o-Me)2,D-Har8]加压素(II),[L-Phe(o-Et)2,D-Har8]加压素(III)和[D-Phe(o-Et)2,D-Har8]加压素(IV)已经合成。所有类似物的抗利尿活性非常低。类似物I和IV是低血压抑制剂。所有类似物都被发现是子宫张力素抑制剂,其中体外抑制作用最强的是[D-Phe(o-Et)2,D-Har8]加压素,其pA2 = 8.4。DOI:10.1135/cccc19921103
文献信息
-
用于合成甲苯二氨基甲酸烷基酯的苯并三唑离 子液体
-
Structure-guided optimization of 1H-imidazole-2-carboxylic acid derivatives affording potent VIM-Type metallo-β-lactamase inhibitors作者:Yu-Hang Yan、Wenfang Li、Wei Chen、Chao Li、Kai-Rong Zhu、Ji Deng、Qing-Qing Dai、Ling-Ling Yang、Zhenling Wang、Guo-Bo LiDOI:10.1016/j.ejmech.2021.113965日期:2022.1Production of metallo-β-lactamases (MBLs) in bacterial pathogens is an important cause of resistance to the ‘last-resort’ carbapenem antibiotics. Development of effective MBL inhibitors to reverse carbapenem resistance in Gram-negative bacteria is still needed. We herein report X-ray structure-guided optimization of 1H-imidazole-2-carboxylic acid (ICA) derivatives by considering how to engage with the active-site细菌病原体中金属-β-内酰胺酶 (MBL) 的产生是对“最后手段”碳青霉烯类抗生素产生耐药性的重要原因。仍然需要开发有效的 MBL 抑制剂来逆转革兰氏阴性菌对碳青霉烯类的耐药性。我们在此报告了 1 H的 X 射线结构引导优化- 咪唑-2-羧酸(ICA)衍生物,通过考虑如何与活性位点柔性环结合并提高对革兰氏阴性细菌的渗透。构效关系研究揭示了在 ICA 1 位适当的取代基对于实现对 B1 类 MBLs,特别是 Verona Integron 编码的 MBLs (VIMs) 的有效抑制的重要性,主要是通过与灵活的活性位点环的巧妙相互作用,如观察到的那样晶体学分析。在测试的 ICA 抑制剂中,55种与美罗培南对工程大肠杆菌菌株甚至难治的临床分离的铜绿假单胞菌具有最强的协同抗菌活性生产 VIM-2 MBL。处理后细菌细胞的形态和内部结构变化进一步证明55穿过外膜并逆转美罗培南的活性。此外,55在体内
-
Novel 4-phenylpiperidines for the treatment of pruritic dermatoses申请人:——公开号:US20030078282A1公开(公告)日:2003-04-24Novel compounds having general formula (I), and pharmaceutically and veterinarily acceptable salts thereof wherein R 1, R 2, R 3, W, Y 1, Y 2, X, n and y are as defined above and processes for their preparation and intermediate compounds prepared therein. The novel compounds are useful for having utility in the treatment. of pruritic dermatoses including allergic dermatitis and atopy in animals and humans具有通用公式(I)的新化合物,以及药物和兽药可接受的盐,其中R 1、R 2、R 3、W、Y 1、Y 2、X、n和y如上所述定义,以及它们的制备过程和其中制备的中间化合物。这些新化合物在治疗包括动物和人类的瘙痒性皮肤病,包括过敏性皮炎和异位症方面具有用途。
-
A General, Activator-Free Palladium-Catalyzed Synthesis of Arylacetic and Benzoic Acids from Formic Acid作者:Lin Wang、Helfried Neumann、Matthias BellerDOI:10.1002/anie.201802384日期:2018.6.4carboxylative synthesis of arylacetic and benzoic acids using formic acid (HCOOH) as the CO surrogate was developed. In an improvement over previous work, CO is generated in situ without the need for any additional activators. Key to success was the use of a specific system consisting of palladium acetate and 1,2‐bis((tert‐butyl(2‐pyridinyl)phosphinyl)methyl)benzene. The generality of this method is demonstrated
-
Identification and Optimization of the First Highly Selective GLUT1 Inhibitor BAY-876作者:Holger Siebeneicher、Arwed Cleve、Hartmut Rehwinkel、Roland Neuhaus、Iring Heisler、Thomas Müller、Marcus Bauser、Bernd BuchmannDOI:10.1002/cmdc.201600276日期:2016.10.19these transporters should not be addressed by such an inhibitor. A high-throughput screen against a library of ∼3 million compounds was performed to find a small molecule with this challenging potency and selectivity profile. The N-(1H-pyrazol-4-yl)quinoline-4-carboxamides were identified as an excellent starting point for further compound optimization. After extensive structure-activity relationship explorations尽管众所周知的事实是,即使在正常的氧气供应条件下,促进性葡萄糖转运蛋白GLUT1仍是保证许多肿瘤实体葡萄糖消耗增加的关键因素之一(被称为Warburg效应),但仅进行了很少的努力寻找一种GLUT1选择性小分子抑制剂。由于GLUT1家族的其他转运蛋白都参与关键过程,因此此类抑制剂不应解决这些转运蛋白。针对约300万种化合物的库进行了高通量筛选,以发现具有这种具有挑战性的效能和选择性的小分子。N-(1H-吡唑-4-基)喹啉-4-羧酰胺被确定为进一步优化化合物的理想起点。经过广泛的构效关系探索后,获得了对GLUT2,GLUT3和GLUT4的选择性因子> 100的个位数纳摩尔抑制剂。最有前途的化合物BAY-876 [N4- [1-(4-氰基苄基)-5-甲基-3-(三氟甲基)-1H-吡唑-4-基] -7-氟喹啉-2,4-二甲酰胺]在体外具有良好的代谢稳定性,在体内具有较高的口服生物利用度。
表征谱图
-
氢谱1HNMR
-
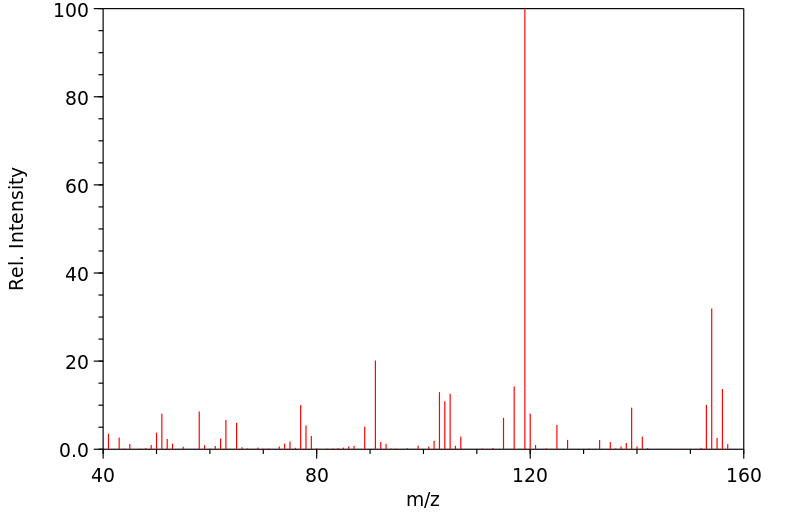
质谱MS
-
碳谱13CNMR
-
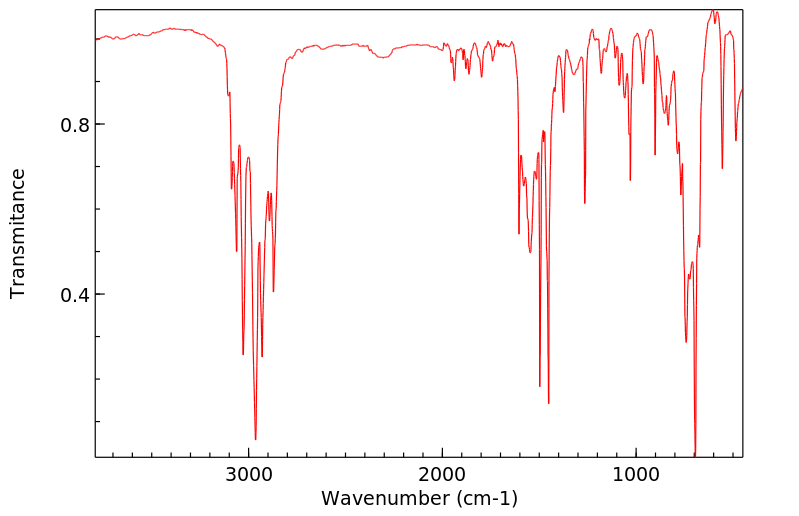
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫