4-异丙基吡啶 | 696-30-0
中文名称
4-异丙基吡啶
中文别名
——
英文名称
4-s-propylpyridine
英文别名
4-isopropylpyridine;4-propan-2-ylpyridine
CAS
696-30-0
化学式
C8H11N
mdl
MFCD00039719
分子量
121.182
InChiKey
FRGXNJWEDDQLFH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-54.9°C
-
沸点:178 °C
-
密度:0,93 g/cm3
-
闪点:66°C
-
LogP:2.330 (est)
-
保留指数:1000;1034
-
稳定性/保质期:
远离氧化物和火源。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.375
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险类别码:R20/21/22,R36/37/38
-
海关编码:2933399090
-
安全说明:S26,S36/37/39
-
储存条件:存放在密封容器中,并置于阴凉、干燥处。存储地点须远离氧化剂和火源。
SDS
4-Isopropylpyridine Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-Isopropylpyridine
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Combustible liquid
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-Isopropylpyridine
Percent: >98.0%(GC)(T)
696-30-0
CAS Number:
Chemical Formula: C8H11N
4-Isopropylpyridine
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
4-Isopropylpyridine
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Very pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 178°C
Flash point: 66°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.93
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Open flame
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
4-Isopropylpyridine
Section 12. ECOLOGICAL INFORMATION
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-Isopropylpyridine
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 4
Flammable liquids
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Combustible liquid
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Keep away from flames and hot surfaces.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-Isopropylpyridine
Percent: >98.0%(GC)(T)
696-30-0
CAS Number:
Chemical Formula: C8H11N
4-Isopropylpyridine
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from flames and hot surfaces. Take
measures to prevent the build up of electrostatic charge. Use explosion-proof
equipment. Wash hands and face thoroughly after handling.
Use a closed system, ventilation.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Storage conditions:
Store under inert gas.
Store away from incompatible materials such as oxidizing agents.
Air-sensitive
Comply with laws.
Packaging material:
4-Isopropylpyridine
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Very pale yellow
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 178°C
Flash point: 66°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.93
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Conditions to avoid: Open flame
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
4-Isopropylpyridine
Section 12. ECOLOGICAL INFORMATION
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
4-异丙基吡啶用作合成双(烷基吡啶)的试剂。而双(烷基吡啶)具有抗真菌和细胞毒性。
制备方法-
在0℃下,向搅拌的5.1 g(14.3 mmol)PbPhCH3Br在25 mL THF中的混合物中,在20分钟内滴加11.0 mL n-BuLi(1.6 M己烷溶液)。1小时后,将1.5 g(12.8 mmol)的1-(吡啶-4-基)乙酮加入20 mL的THF中。将混合物在0℃下搅拌1小时,然后在室温下搅拌50分钟。将混合物通过布氏漏斗过滤。用饱和NH4Cl和H2O处理,分离各层。将有机层用盐水洗涤,用Na2SO4干燥,过滤并浓缩。通过快速硅胶色谱法(54%EtOAc/己烷)纯化,得到异丙烯基吡啶,为浅黄色液体。
-
在室温下,在H2气球下搅拌4-(丙-1-烯-2-基)吡啶和342 mg 20%Pd(OH)2在15 mL EtOAc和10 mL MeOH中的混合物。24小时后,加入305 mg 20%Pd(OH)2,6小时后,将混合物通过硅藻土过滤,过滤并浓缩。将粗产物溶于15 mL MeOH中,并加入512 mg 20%Pd(OH)2。将混合物在H2气球下在室温下搅拌11.5小时,通过硅藻土过滤,并浓缩,得到4-异丙基吡啶。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-乙基吡啶 4-Ethylpyridine 536-75-4 C7H9N 107.155 2-(4-吡啶基)-2-丙醇 2-(4-pyridyl)propan-2-ol 15031-78-4 C8H11NO 137.181 —— 4-(prop-1-en-2-yl)pyridine 17755-30-5 C8H9N 119.166 4-甲基吡啶 picoline 108-89-4 C6H7N 93.1283 4-乙酰吡啶 methyl (4-pyridyl) ketone 1122-54-9 C7H7NO 121.139 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-叔丁基吡啶 4-t-butylpyridine 3978-81-2 C9H13N 135.209 4-乙基吡啶 4-Ethylpyridine 536-75-4 C7H9N 107.155 4-异丙基吡啶氮氧化物 4-isopropylpyridine 1-oxide 22581-87-9 C8H11NO 137.181 2-(4-吡啶基)-2-丙醇 2-(4-pyridyl)propan-2-ol 15031-78-4 C8H11NO 137.181 2-甲基-2-(4-吡啶)-1-丙醇 2-methyl-2-(4-pyridyl)-1-propanol 34995-28-3 C9H13NO 151.208 —— 2-(4-Pyridyl)-2-chlorpropan 40473-14-1 C8H10ClN 155.627 —— 4-(prop-1-en-2-yl)pyridine 17755-30-5 C8H9N 119.166 4-乙酰吡啶 methyl (4-pyridyl) ketone 1122-54-9 C7H7NO 121.139 4-甲基吡啶 picoline 108-89-4 C6H7N 93.1283 2-氟-4-异丙基吡啶 2-fluoro-4-isopropylpyridine 111887-69-5 C8H10FN 139.173 2-氯-4-异丙基吡啶 2-chloro-4-isopropylpyridine 959020-16-7 C8H10ClN 155.627 —— 4-(1,1-dimethyl-2-(methylthio)ethyl)pyridine 103905-24-4 C10H15NS 181.302 - 1
- 2
反应信息
-
作为反应物:描述:4-异丙基吡啶 在 N-羟基-7-氮杂苯并三氮唑 、 iron(II) tetrafluoroborate hexahydrate 、 氧气 、 三吡唑啉基硼氢钾 作用下, 以 苯甲腈 为溶剂, 反应 18.0h, 以17.8 mg的产率得到4-乙酰吡啶参考文献:名称:用于氮杂杂环附近位点选择性氧化的复合铁/羟基三唑双催化体系摘要:本报告详细介绍了一种与氮杂杂环相邻的位点选择性亚甲基氧化的新方法。双催化方法,利用铁路易斯酸和有机羟胺催化剂,证明是非常有效的。我们证明该方法为其他已知的催化方法提供了互补的选择性,并代表了对目前依赖化学计量活化的杂环选择性反应的改进。DOI:10.1021/jacs.7b12864
-
作为产物:参考文献:名称:Caccia, G.; Chelucci, G.; Botteghi, C., Synthetic Communications, 1981, vol. 11, # 1, p. 71 - 84摘要:DOI:
-
作为试剂:描述:参考文献:名称:聚合物负载的钴催化芳基炔的区域选择性环三聚反应摘要:开发了一种复杂的聚(4-乙烯基吡啶)钴(II)(P4VP-CoCl 2 )体系作为稳定且可重复使用的多相催化剂。根据实验数据和理论计算确定了Co原子附近的局域结构。该固定化钴催化剂在末端芳基炔烃的[2+2+2]环三聚反应中表现出高选择性和催化活性。使用0.033 mol% P4VP-CoCl 2 ,实现了1,3,5-三芳基苯的区域选择性形成,而没有形成1,2,4-三芳基苯。此外,数克规模(11g)的反应有效地进行。此外,聚合物负载催化剂已成功回收并使用3次。回收的催化剂的X射线光电子能谱分析表明钴处于+2氧化态。 1,3,5-三芳基苯衍生物应用于分子束电子抗蚀剂和多环芳烃的合成。DOI:10.1021/jacsau.1c00360
文献信息
-
Integrase inhibitors申请人:Cai R. Zhenhong公开号:US20080058315A1公开(公告)日:2008-03-06Tricyclic compounds, protected intermediates thereof, and methods for inhibition of HIV-integrase are disclosed.三环化合物,其受保护的中间体,以及用于抑制HIV整合酶的方法被披露。
-
SUBSTITUTED BRIDGED UREA ANALOGS AS SIRTUIN MODULATORS申请人:GLAXOSMITHKLINE LLC公开号:US20150152108A1公开(公告)日:2015-06-04The present invention relates to novel substituted bridged urea compounds, corresponding related analogs, pharmaceutical compositions and methods of use thereof. Sirtuin-modulating compounds of the present invention may be used for increasing the lifespan of a cell, and treating and/or preventing a wide variety of diseases and disorders, which include, but are not limited to, for example, diseases or disorders related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders, inflammation, cancer, and/or flushing as well as diseases or disorders that would benefit from increased mitochondrial activity. The present invention also related to compositions comprising a sirtuin-modulating compound in combination with another therapeutic agent.本发明涉及新型取代桥式脲化合物,相应的相关类似物,药物组合物以及其使用方法。本发明的抑制素调节化合物可用于延长细胞寿命,并治疗和/或预防各种疾病和疾病,包括但不限于与衰老或压力、糖尿病、肥胖、神经退行性疾病、心血管疾病、血液凝块疾病、炎症、癌症和/或潮红有关的疾病或疾病,以及那些会受益于增加线粒体活性的疾病或疾病。本发明还涉及包含抑制素调节化合物与另一治疗剂组合的组合物。
-
COMPOUNDS AND METHODS FOR THE TARGETED DEGRADATION OF INTERLEUKIN-1 RECEPTOR-ASSOCIATED KINASE 4 POLYPEPTIDES申请人:Arvinas, Inc.公开号:US20190151295A1公开(公告)日:2019-05-23The present disclosure relates to bifunctional compounds, which find utility as modulators of Interleukin-1 Receptor-Associated Kinase 4 (IRAK-4); the target protein). In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a Von Hppel-Lindau, cereblon, ligand which binds to the E3 ubiquitin ligase and on the other end a moiety which binds the target protein, such that the target protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of target protein. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of target protein. Diseases or disorders that result from aggregation or accumulation of the target protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为白细胞介素-1受体相关激酶4(IRAK-4;目标蛋白)的调节剂具有实用性。具体而言,本公开涉及包含一端结合E3泛素连接酶的Von Hppel-Lindau、cereblon配体的双功能化合物,另一端结合目标蛋白的部分,使得目标蛋白靠近泛素连接酶以实现目标蛋白的降解(和抑制)。本公开展示了与目标蛋白的降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由目标蛋白聚集或积累导致的疾病或紊乱。
-
COMPOUNDS AND USES THEREOF申请人:Yumanity Therapeutics, Inc.公开号:US20190330198A1公开(公告)日:2019-10-31The present invention features compounds useful in the treatment of neurological disorders. The compounds of the invention, alone or in combination with other pharmaceutically active agents, can be used for treating or preventing neurological disorders.本发明涉及用于治疗神经系统疾病的化合物。本发明的化合物可以单独或与其他药用活性剂结合使用,用于治疗或预防神经系统疾病。
-
SUBSTITUTED HETEROCYCLES AS ANTIVIRAL AGENTS申请人:Enanta Pharmaceuticals, Inc.公开号:US20190224188A1公开(公告)日:2019-07-25The present invention discloses compounds of Formula (I), and pharmaceutically acceptable salts thereof: which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.本发明公开了式(I)的化合物及其药学上可接受的盐: 这些化合物抑制由乙型肝炎病毒(HBV)编码的蛋白质或干扰乙型肝炎病毒的生命周期功能,并且还可用作抗病毒剂。本发明还涉及包括上述化合物的药物组合物,用于治疗患有HBV感染的受试者。该发明还涉及通过给予包含本发明化合物的药物组合物来治疗受试者的HBV感染的方法。
表征谱图
-
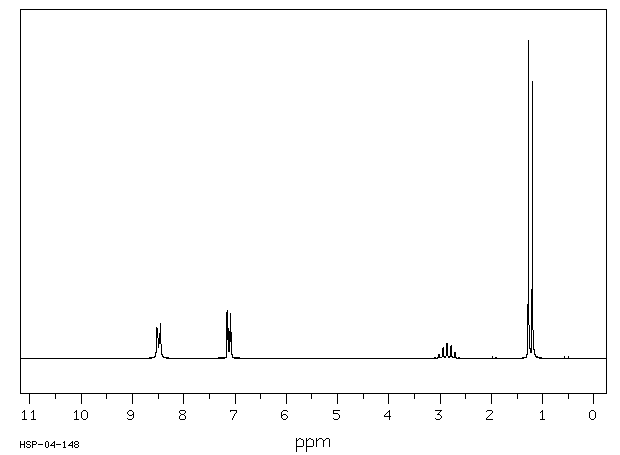
氢谱1HNMR
-
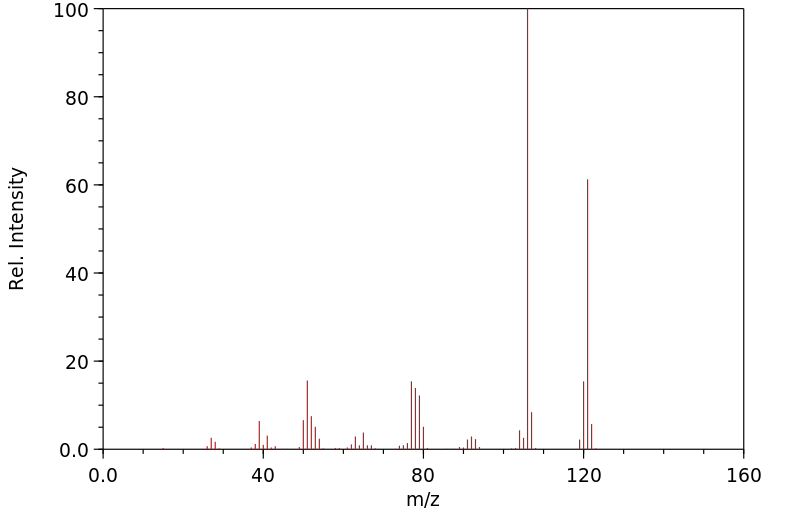
质谱MS
-
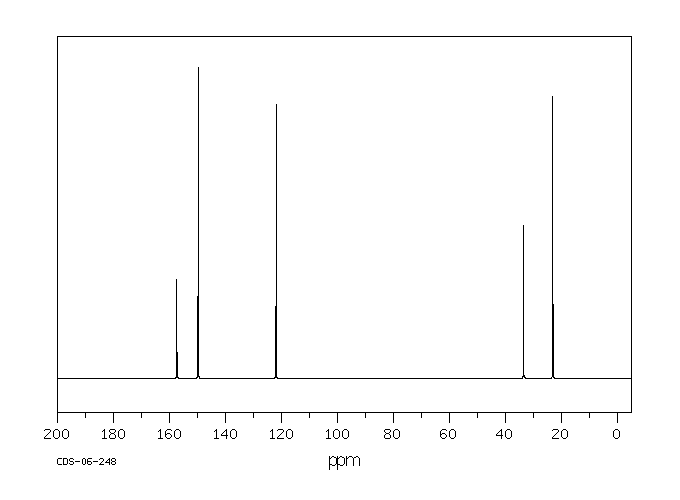
碳谱13CNMR
-
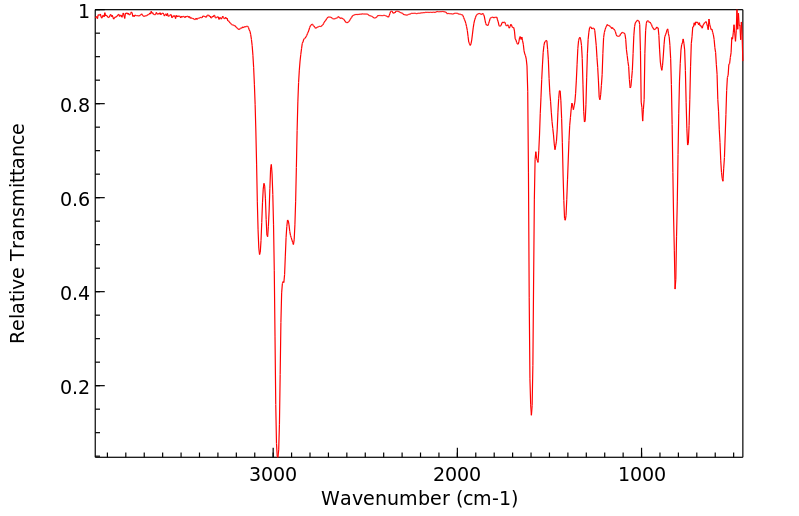
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-