3,7,7-trimethyl-cyclohepta-1,3,5-triene | 3479-89-8
中文名称
——
中文别名
——
英文名称
3,7,7-trimethyl-cyclohepta-1,3,5-triene
英文别名
3,7,7‐trimethyl‐1,3,5‐cycloheptatriene;3,7,7-trimethyl-1,3,5-cycloheptatriene;3,7,7-trimethylcyclohepta-1,3,5-triene;1,1,4-trimethylcycloheptatriene;3,7,7-Trimethylcycloheptatriene;3,7,7-trimethyltropilidene
CAS
3479-89-8
化学式
C10H14
mdl
——
分子量
134.221
InChiKey
VOURXYUVXXLJMD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:65 °C(Press: 70 Torr)
-
密度:0.8545 g/cm3
-
保留指数:972.6;980;953;970
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,7,7-Trimethyl-tropiliden 2228-74-2 C10H14 134.221
反应信息
-
作为反应物:参考文献:名称:Thermally Induced Skeletal Rearrangements of Tropilidenes1摘要:DOI:10.1021/ja00963a025
-
作为产物:描述:反应 0.5h, 生成 3,7,7-trimethyl-cyclohepta-1,3,5-triene参考文献:名称:Deleris, G.; Gadras, A.; Dunogues, J., Phosphorus, Sulfur and Silicon and the Related Elements, 1995, vol. 101, # 1-4, p. 287 - 290摘要:DOI:
文献信息
-
Identification by GC—MS of cymene isomers and 3,7,7-trimethylcyclohepta-1,3,5-triene in essential oils作者:E. P. Romanenko、A. V. TkachevDOI:10.1007/s10600-006-0256-6日期:2006.11Retention indices of cymene isomers published in popular GC—MS atlases were found to be erroneous by analyzing synthetic samples. The following retention indices (RI) were found using a nonpolar phase (diphenyl: dimethylpolysiloxane, 5:95) for four essential-oil components with indistinguishable mass spectra: 3,7,7-trimethylcyclohepta-1,3,5-triene (RI = 970), m-cymene (RI = 1022), p-cymene (RI = 1024), and o-cymene (RI = 1039). The relative distributions of these compounds were evaluated based on the analysis of about 1000 essential oils. Simple methods were given for preparing standard mixtures of isomeric compounds for identification by GC—MS.
-
Organic reactions in a solid matrix—III作者:V.S. Joshi、N.P. Damodaran、Sukh DevDOI:10.1016/s0040-4020(01)90717-3日期:1971.11-Methyl-1,2-epoxycycloheptane, 2α,3α-epoxypinane and 3α,4α-epoxycarane are shown to undergo typical carbonium ion rearrangements in contact with active silica gel. Humulene epoxide-II and caryophyllene oxide did not undergo any transformation under these conditions. All these results are in complete contrast to their behaviour on exposure to alumina.
-
Reactions of Coordinated cyclic polyolefins. The synthesis and cycloaddition reactions of tricarbonyl[(1,2,3,4-η)-3,7,7-trimethylcycloheptatriene]iron作者:Zeev Goldschmidt、Shlomo AntebiDOI:10.1016/0022-328x(83)85162-6日期:1983.12regiospecificity of the reactions is considered in terms of frontier molecular orbitals. Complex III reacts with tetracyanoethylene to form a mixture of 1,3- and 1,6-cycloadducts in 4/1 ratio. Diphenylketene (DPK) forms a 2 + 2 adduct with III at room temperature. At 80°C an acylated addition product is obtained. In general, steric hindrance increases the yield of products formed via bipolar reactions at the expense
-
Campbell et al., Journal of the Chemical Society, 1956, p. 4096,4100作者:Campbell et al.DOI:——日期:——
-
The Stereochemistry of the α-Amyrins作者:Elias J. Corey、Joseph J. UrsprungDOI:10.1021/ja01582a052日期:1956.1
表征谱图
-
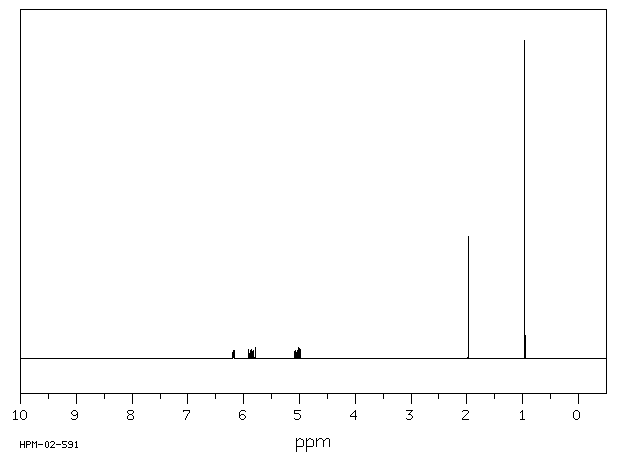
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸