5-(4-氟苯基)-4,5-二氢-2(3H)-呋喃酮 | 51787-96-3
中文名称
5-(4-氟苯基)-4,5-二氢-2(3H)-呋喃酮
中文别名
4,5-二氢-5-(4-氟苯基)-2(3H)-呋喃酮;gamma-(4-氟苯基)-gamma-丁内酯;4-(4-氟苯基)丁内酯;5-(4-氟苯基)-二氢-2(3H)-呋喃酮;Γ-(4-氟苯基)-Γ-丁内酮
英文名称
5-(4-fluorophenyl)dihydrofuran-2(3H)-one
英文别名
γ-(4-fluorophenyl)-γ-butyrolactone;5-(4-fluorophenyl)dihydrofuran-2-one;5-(p-fluorophenyl)tetrahydro-2-furanone;gamma-(4-Fluorophenyl)-gamma-butyrolactone;5-(4-fluorophenyl)oxolan-2-one
CAS
51787-96-3
化学式
C10H9FO2
mdl
——
分子量
180.179
InChiKey
RMFNZGXVLAUJHF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:324.5±42.0 °C(Predicted)
-
密度:1.2000
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:F,Xi
-
WGK Germany:3
-
海关编码:29031980
-
危险类别:3
-
安全说明:S29,S9
-
危险类别码:R36/37/38,R11
-
包装等级:I
-
危险品运输编号:UN 2456 3/PG 1
-
储存条件:存放于阴凉干燥处即可。
SDS
| Name: | gamma-(4-Fluorophenyl)-gamma-butyrolactone 98% Material Safety Data Sheet |
| Synonym: | 5-(4-Fluorophenyl)-dihydro-2(3H)-furanon |
| CAS: | 51787-96-3 |
Synonym:5-(4-Fluorophenyl)-dihydro-2(3H)-furanon
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 51787-96-3 | gamma-(4-Fluorophenyl)-gamma-butyrolac | 98 | 257-422-5 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 51787-96-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear, colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: viscous
Boiling Point: 129.0 - 131.0 deg C @ 1.00mba
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: 1.2000g/cm3
Molecular Formula: C10H9FO2
Molecular Weight: 180.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 51787-96-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
gamma-(4-Fluorophenyl)-gamma-butyrolactone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 51787-96-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 51787-96-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 51787-96-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:医药中间体,具体为五氟利多的中间体。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (R)-5-(4-fluorophenyl)dihydrofuran-2(3H)-one 75738-79-3 C10H9FO2 180.179 —— (S)-5-(4-fluorophenyl)dihydrofuran-2(3H)-one 75738-80-6 C10H9FO2 180.179 —— 5-(4-fluorophenyl)-3-methylenedihydrofuran-2(3H)-one 147596-64-3 C11H9FO2 192.19 —— cis/trans-5-(4-Fluorphenyl)-2-oxo-4,5-dihydro-3H-furan-3-carbonsaeure 147596-60-9 C11H9FO4 224.188 —— trans-5R-5-(4-fluorophenyl)-2-hydroxy tetrahydrofuran 175415-52-8 C10H11FO2 182.195 —— 5-(4-fluorophenyl)tetrahydrofuran-2-ol 75738-81-7 C10H11FO2 182.195 —— dl-4-hydroxy-4-(4-fluorophenyl)butyric acid 87545-51-5 C10H11FO3 198.194
反应信息
-
作为反应物:参考文献:名称:五氟利多的制备方法摘要:本发明公开了一种五氟利多的制备方法,包括如下步骤:(1)将丁二酸酐和氟苯经付氏反应,再用酸分解,收集式(2)化合物;(2)然后在溶剂中,经还原剂还原,收集式(3)化合物;(3)再与氟苯经Friedel–Crafts反应,收集式(4)化合物,(4)然后与氯甲酸乙酯反应,生成式(5)化合物,(5)与式(ⅩⅦ)所示化合物反应,水解,收集式(6)化合物,(6)用还原剂还原,再对还原产物进行分解,然后收集五氟利多(Ⅰ);本发明收率高,成本低,反应条件温和,路线短,适合工业生产,三废少且易处理,适用于工业生产。反应通式如下。公开号:CN106187863A
-
作为产物:描述:氟苯 在 aluminum (III) chloride 、 C12H14N4*Ir(1+)*2CO*BF4(1-)*C3H7NO 、 氢气 、 potassium hydroxide 作用下, 以 异丙醇 为溶剂, 100.0 ℃ 、5.07 MPa 条件下, 反应 10.0h, 生成 5-(4-氟苯基)-4,5-二氢-2(3H)-呋喃酮参考文献:名称:通过使用NHC-铱配位聚合物作为固体分子催化剂将生物质含氧酸有效氢化为内酯摘要:事实证明,一系列NHC-铱配位聚合物对于将生物质乙酰丙酸(LA)加氢成γ-戊内酯是一种坚固,高效且可回收的固体分子催化剂。除了在50个大气压的H 2下以0.01 mol%的催化剂负载量获得定量收率外,固体分子催化剂也易于回收并重复使用12次,而选择性和活性没有明显损失。值得注意的是, 在这一重要的转变中,高达1.2×10 5 TON可以实现空前的价值。另外,许多LA同系物,类似物和衍生物具有良好的耐受性,以良好至优异的产率提供了各种有趣的和功能性的内酯,这进一步证实了固体分子催化剂的可行性。DOI:10.1002/asia.201601537
文献信息
-
Metal‐Free Synthesis of <i>N</i> ‐Aryl Amides using Organocatalytic Ring‐Opening Aminolysis of Lactones作者:Wusheng Guo、José Enrique Gómez、Luis Martínez‐Rodríguez、Nuno A. G. Bandeira、Carles Bo、Arjan W. KleijDOI:10.1002/cssc.201700415日期:2017.5.9Catalytic ring‐opening of bio‐sourced non‐strained lactones with aromatic amines can offer a straightforward, 100 % atom‐economical, and sustainable pathway towards relevant N‐aryl amide scaffolds. Herein, the first general, metal‐free, and highly efficient N‐aryl amide formation is reported from poorly reactive aromatic amines and non‐strained lactones under mild operating conditions using an organic
-
Efficient hydrogenation of levulinic acid catalysed by spherical NHC-Ir assemblies with atmospheric pressure of hydrogen作者:Lingyun Shen、Qingshu Zheng、Yaoqi Liu、Jiajie Wu、Zeye Lu、Tao TuDOI:10.1039/d1gc01513c日期:——
Enhanced catalytic activity towards hydrogenation of levulinic acid to γ-valerolactone under 1 atm H2 was realized by spherical porous self-supported NHC-Ir catalysts.
-
MnO<sub>2</sub>-promoted carboesterification of alkenes with anhydrides: a facile approach to γ-lactones作者:Lihuan Wu、Zhenming Zhang、Jianhua Liao、Jianxiao Li、Wanqing Wu、Huanfeng JiangDOI:10.1039/c5cc08867d日期:——
A new radical cyclization method for the formation of C(sp3)–C(sp3) and C–O bonds
via MnO2-promoted alkene carboesterification with anhydrides is developed.一种新的基于锰促进的烯烃碳酯化反应的C(sp3)-C(sp3)和C-O键的放射状环化方法被开发出来。 -
Catalytic Redox Chain Ring Opening of Lactones with Quinones To Synthesize Quinone-Containing Carboxylic Acids作者:Xiao-Long Xu、Zhi LiDOI:10.1021/acs.orglett.9b01672日期:2019.7.5Catalytic ring opening of five- to eight-membered lactones with quinones is achieved through a redox chain mechanism. With low loading of a simple metal triflate Lewis acid catalyst and a chain initiator, C–H bonds of many quinones were efficiently functionalized with carboxylic acid-containing side chains. This method also features 100% atom economy and wide substrate scope. A novel route to the anti-asthma
-
Cooperative iodine and photoredox catalysis for direct oxidative lactonization of carboxylic acids作者:Thomas Duhamel、Kilian MuñizDOI:10.1039/c8cc08594c日期:——A new method for the formation of γ- and δ-lactones from carboxylic acids through direct conversion of benzylic C–H to C–O bonds is described. The reaction is conveniently induced by visible light and relies on a mild cooperative catalysis by the combination of molecular iodine and an organic dye.
表征谱图
-
氢谱1HNMR
-
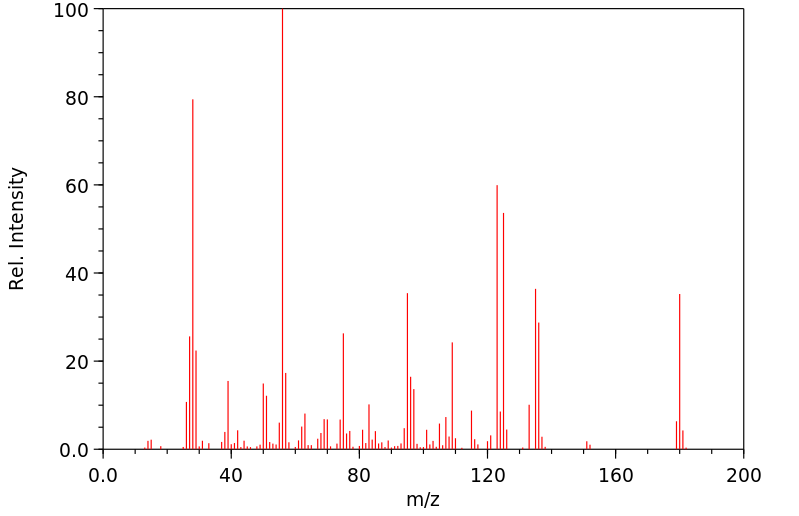
质谱MS
-
碳谱13CNMR
-
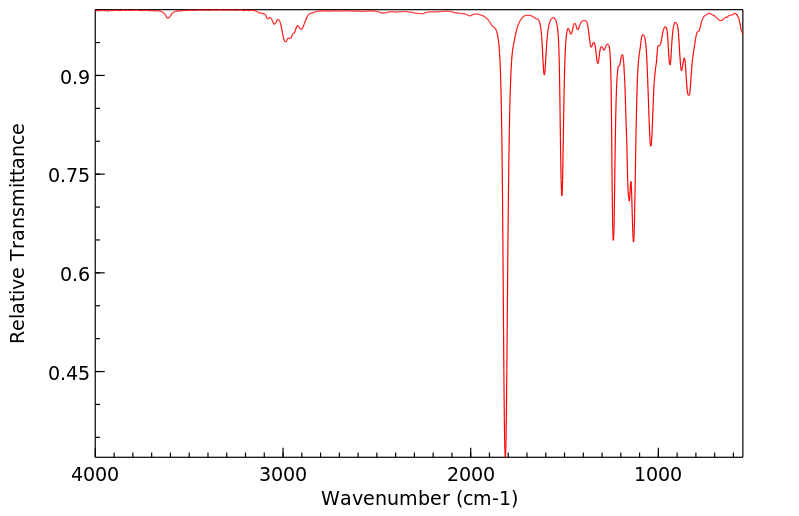
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫