5-甲基-5-己烯-2-醇 | 50551-88-7
中文名称
5-甲基-5-己烯-2-醇
中文别名
——
英文名称
5-methylhex-5-en-2-ol
英文别名
5-methyl-5-hexen-2-ol;2-Methyl-hexen-(1)-ol-(5)
CAS
50551-88-7
化学式
C7H14O
mdl
——
分子量
114.188
InChiKey
RIEDLCPIEKXTTL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:73-74 °C(Press: 26.5-28.5 Torr)
-
密度:0.834±0.06 g/cm3(Predicted)
-
LogP:1.548 (est)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
反应信息
-
作为反应物:描述:5-甲基-5-己烯-2-醇 在 吡啶 、 2-萘硫醇 作用下, 以 氘代苯 、 N,N-二甲基甲酰胺 为溶剂, 反应 14.08h, 生成 2,2,5-Trimethyl-tetrahydro-furan参考文献:名称:Ring Closure Reactions of Substituted 4-Pentenyl-1-oxy Radicals. The Stereoselective Synthesis of Functionalized Disubstituted Tetrahydrofurans摘要:N-(Alkyloxy)pyridine-2( VT)-thiones 3 and benzenesulfenic acid O-esters 5 have been synthesized from substituted 4-pentenols 1 or the derived tosylates. Compounds 3 and 5 are efficient sources of free alkoxy radicals 6 which undergo synthetically useful fast ring closure reactions 6 --> 8 [k(exo) = (2 +/- 1) x 10(8) s(-1) to (6 +/- 2) x 10(9) s(-1) (T = 30 +/- 0.2 degrees C)]. Tetrahydrofurfuryl radicals 8 can be trapped with, e.g., hydrogen or chlorine atom donors to afford either trans- or cis-disubstituted tetrahydrofurans 10 or 12 depending on the substitution pattern of the 4-pentenyloxy radical. Substituted tetrahydropyrans 11 or 13 are formed in the minor 6-endo-trig cyclization. According to the data of competition kinetics, the observed stereoselectivities in free alkoxy radical cyclizations arise from steric interactions between the substituents in the transition state of the ring closure reactions. Alkyl substituents cause small differences in the measured relative rate constants of B-exo cyclizations which are reminiscent of the data obtained from the rearrangements of alkyl-substituted 5-hexenyl radicals. Likewise, a stereochemical model for oxygen radical cyclization is proposed where the pentenyloxy chain adopts a six-membered, chairlike transition state with the alkyl substituents preferentially situated in the pseudoequatorial positions leading to 2,5-trans-, 2,4-cis-, and 2,3-trans-substituted tetrahydrofurfuryl radicals 8 as the major intermediates.DOI:10.1021/jo00126a021
-
作为产物:描述:参考文献:名称:VIII族金属催化剂在非共轭烯酮加氢中的化学选择性摘要:VIII族金属催化剂的化学选择性已在常温氢气压力下非共轭烯酮的氢化中得到检验。钴催化剂从三烷基化烯烃酮中得到高收率的不饱和醇。在铂金属催化剂中,锇在三烷基化烯烃酮的加氢反应中对羰基键的还原显示出最高的选择性。无论催化剂如何,单烷基化和二烷基化烯烃酮的氢化通常以烯烃官能团的优先饱和进行。4-亚甲基-和4-亚乙基环己酮的氢化伴随着在乙醇溶剂中在钌、铑和钯黑催化剂上形成二乙缩醛。在其他烯酮的氢化和其他催化剂上未检测到缩醛。此外,还研究了镍和钴催化剂对无环烯酮加氢的化学选择性。DOI:10.1246/bcsj.60.1721
文献信息
-
Cooperative Mn(<scp>i</scp>)-complex catalyzed transfer hydrogenation of ketones and imines作者:Kasturi Ganguli、Sujan Shee、Dibyajyoti Panja、Sabuj KunduDOI:10.1039/c8dt05001e日期:——complex presented higher reactivity in the transfer hydrogenation (TH) of ketones in 2-propanol. Experimentally, it was established that both the benzimidazole and amine N–H proton played a vital role in the enhancement of the catalytic activity. Utilizing this system a wide range of aldehydes and ketones were reduced efficiently. Notably, the TH of several imines, as well as chemoselective reduction of
-
6,6′-Dihydroxy terpyridine: a proton-responsive bifunctional ligand and its application in catalytic transfer hydrogenation of ketones作者:Cameron M. Moore、Nathaniel K. SzymczakDOI:10.1039/c2cc36927c日期:——The ligand 6,6â²-dihydroxy terpyridine (dhtp) is presented as a bifunctional ligand capable of directing proton transfer events with metal-coordinated substrates. Solid-state analysis of a Ru(II)-dhtp complex reveals directed hydrogen-bonding interactions of the hydroxyl groups of dhtp with a Ru-bound chloride ligand. The utility of dhtp was demonstrated by chemoselective transfer hydrogenation of ketones.
-
METHOD FOR PRODUCING OLEFINICALLY UNSATURATED CARBONYL COMPOUNDS BY OXIDATIVE DEHYDROGENATION OF ALCOHOLS申请人:Limbach Michael公开号:US20110004025A1公开(公告)日:2011-01-06A process for preparing olefinically unsaturated carbonyl compounds by oxidative dehydrogenation in an oxygenous atmosphere over a supported catalyst which comprises gold and optionally further noble metals at temperatures in the range from 50 to 240° C.
-
Compounds capable of activating cholinergic receptors申请人:——公开号:US20030125345A1公开(公告)日:2003-07-03The present invention generally relates to nicotinic compounds, in the form of aryl substituted olefinic compounds, as well as pro-drug, N-oxide, metabolite and pharmaceutically acceptable salt forms thereof. Methods of modulating neurotransmitter release via administration of the compounds, pro-drugs, N-oxides and/or pharmaceutically acceptable salts are also disclosed.本发明通常涉及烟碱化合物,以芳基取代的烯烃化合物形式存在,以及它们的前药、N-氧化物、代谢物和药用可接受盐形式。还公开了通过给予这些化合物、前药、N-氧化物和/或药用可接受盐来调节神经递质释放的方法。
-
5-(Methylthio)tetrazoles as versatile synthons in the stereoselective synthesis of polycyclic pyrazolines via photoinduced intramolecular nitrile imine–alkene 1,3-dipolar cycloaddition作者:Daniel Pla、Derek S. Tan、David Y. GinDOI:10.1039/c4sc00107a日期:——A key thioether substituent in readily accessible 2-alkyl-5-(methylthio)tetrazoles enables facile photoinduced denitrogenation and intramolecular nitrile imine 1,3-dipolar cycloaddition to afford a wide range of polycyclic pyrazoline products with excellent diastereoselectivity. The methylthio group red-shifts the UV absorbance of the tetrazole, obviating the requirement in all previous substrate systems
表征谱图
-
氢谱1HNMR
-
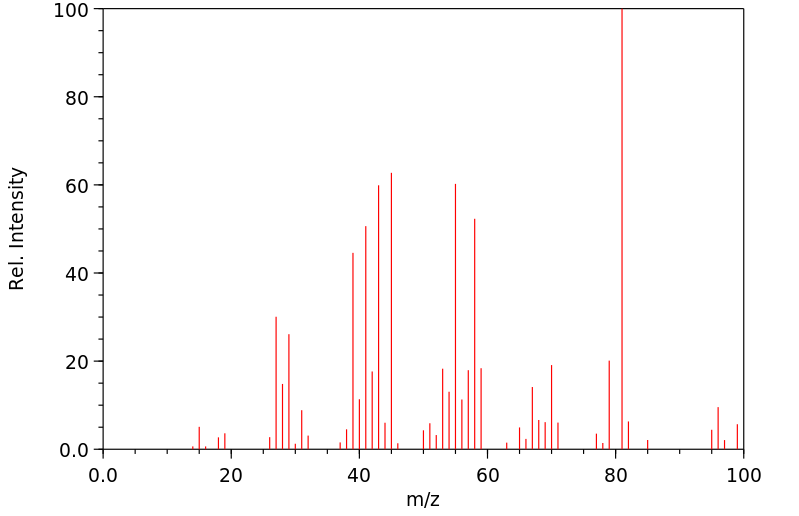
质谱MS
-
碳谱13CNMR
-
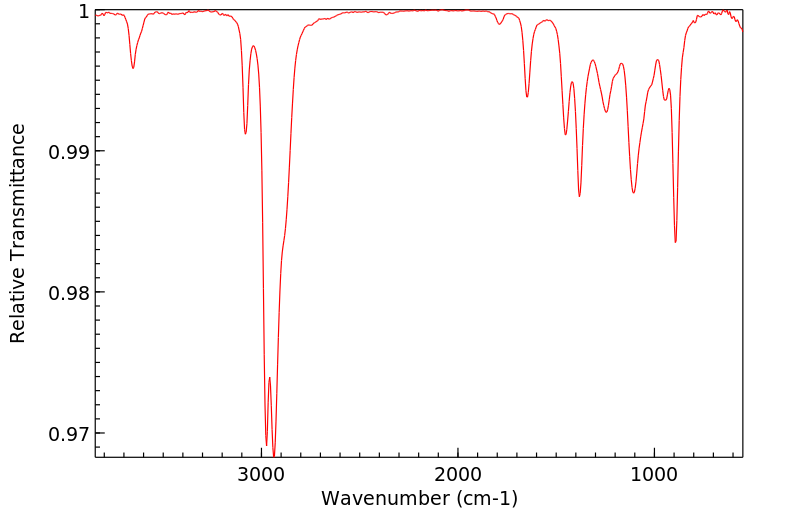
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷