6-双环[2.2.1]庚基乙酸酯 | 34640-76-1
中文名称
6-双环[2.2.1]庚基乙酸酯
中文别名
——
英文名称
2-Norbonylacetat
英文别名
endo-norbornyl acetate;exo-norbornyl acetate;exo-2-norbornyl acetate;norborneol acetate;2-Acetoxymethyl-bicyclo<2.2.1>heptan;2-Acetoxybicyclo<2.2.1>heptan;2-Acetoxynorbornan (exo);norbornyl acetate;2-bicyclo[2.2.1]heptanyl acetate
CAS
34640-76-1
化学式
C9H14O2
mdl
——
分子量
154.209
InChiKey
YXNICIBZSREEPY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
LogP:2.319 (est)
-
保留指数:1114;1114.3;1112;1112
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:11
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915390090
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 2-Norbornyl acetate
CAS-No. : 34640-76-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
Caution - substance not yet tested completely.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Synonyms : bicyclo[2.2.1]hept-2-yl acetate
Formula : C9H14O2
Molecular Weight : 154,21 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Normal measures for preventive fire protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection not required. For nuisance exposures use type OV/AG (US) or type ABEK
(EU EN 14387) respirator cartridges. Use respirators and components tested and approved under
appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation
May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲氧基二环[2.2.1]庚烷 2-methoxybicyclo[2.2.1]heptane 10395-53-6 C8H14O 126.199 5-降冰烯-2-基乙酸酯 5-acetoxynorborn-2-ene 6143-29-9 C9H12O2 152.193 双环[2.2.1]-2-庚醇 Norborneol 1632-68-4 C7H12O 112.172 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 降冰片烷-2-基丙-2-烯酸酯 norbornyl acrylate 10027-06-2 C10H14O2 166.22 甲丙烯酰酸去甲冰片酯 norbornyl (meth)acrylate 29753-02-4 C11H16O2 180.247 双环[2.2.1]-2-庚醇 Norborneol 1632-68-4 C7H12O 112.172 —— (1S,2S,4R)-2-norbornanol 497-37-0 C7H12O 112.172
反应信息
-
作为反应物:描述:参考文献:名称:一种带桥环结构(甲基)丙烯酸酯的制备方法摘要:本发明涉及一种带桥环结构(甲基)丙烯酸酯的制备方法,通过三步反应合成,首先利用饱和脂肪酸与桥环烯烃或其衍生物进行直接加成,精馏提纯,去除桥环烯烃自聚产生的副产物;然后进行水解,得到对应的桥环醇或其衍生物;最后利用(甲基)丙烯酸甲酯与之进行酯交换反应得到带桥环结构(甲基)丙烯酸酯。本发明制备方法简单,步骤易于操作,通过三步反应得到目标产物且收率高,克服了直接酯化工艺精馏过程要求高、收率低的缺点,产品残留环烯烃单体含量低,应用于树脂合成时,可明显改善树脂黄变以及粘度增加的缺点。公开号:CN108299194A
-
作为产物:描述:2-Acetoxy-2-norbornylquecksilberchlorid 在 sodium tetrahydroborate 作用下, 以 二氯甲烷 、 水 为溶剂, 反应 0.5h, 以83%的产率得到6-双环[2.2.1]庚基乙酸酯参考文献:名称:Giese, Bernd; Erfort, Ulrich, Chemische Berichte, 1983, vol. 116, # 3, p. 1240 - 1251摘要:DOI:
文献信息
-
Aluminium dodecatungstophosphate (AlPW12O40) as a highly efficient catalyst for the selective acetylation of –OH, –SH and –NH2 functional groups in the absence of solvent at room temperature作者:Habib Firouzabadi、Nasser Iranpoor、Farhad Nowrouzi、Kamal AmaniDOI:10.1039/b300775h日期:2003.3.6AlPW12O40 was found to be an effective catalyst for the selective acetylation of alcohols, thiols, and amines in the absence of solvent at room temperature.AlPW12O40被发现是一种有效的催化剂,可在室温无溶剂条件下选择性乙酰化醇、硫醇和胺。
-
Consecutive addition esterification and hydrolysis of cyclic olefins catalyzed by multi-SO<sub>3</sub>H functionalized multi heteropolyanion-based ionic hybrids undersolvent-free conditions作者:Guocai Zheng、Xinzhong LiDOI:10.1080/00397911.2019.1580743日期:2019.4.3for the synthesis of cycloalkyl carboxylates and alcohols from cyclic olefins is described. The cyclic olefins were converted to corresponding target molecules under solvent-free conditions catalyzed by two novel multi-SO3H functionalized multi heteropolyanion-based ionic hybrids through one-pot consecutive addition esterification and hydrolysis reactions. This approach has several advantages, including
-
Fluorine-Containing Sulfonic Acid Salt, Fluorine-Containing Sulfonic Acid Salt Resin, Resist Composition, and Pattern Forming Method Using Same申请人:Central Glass Company, Limited公开号:US20150198879A1公开(公告)日:2015-07-16Disclosed is a fluorine-containing sulfonic acid salt resin having a repeating unit represented by the following general formula (3). In the formula, each A independently represents a hydrogen atom, a fluorine atom or a trifluoromethyl group, and n represents an integer of 1-10. W represents a bivalent linking group, R 01 represents a hydrogen atom or a monovalent organic group, and M + represents a monovalent cation. A resist composition containing this resin is further superior in sensitivity, resolution and reproducibility of mask pattern and is capable of forming a pattern with a low LER.
-
Reaction of Organoboranes with Lead(IV) Acetate Azide. A Synthesis of Azidoalkanes from Alkenes<i>via</i>Hydroboration作者:Yuzuru Masuda、Masayuki Hoshi、Akira AraseDOI:10.1246/bcsj.57.1026日期:1984.4Trialkylboranes prepared from alkenes via hydroboration react with lead(IV) acetate azide in dichloromethane at −25 °C to form the corresponding azidoalkanes in a one-pot manner. One or two of the alkyl groups of trialkylboranes are utilized in the reaction. For example, 1-azidohexane is afforded from 1-hexene in 50% yield based on the alkene employed.
-
The novel synthesis of alkyl cyanides by the reaction of sodium trialkylcyanoborate with sodium cyanide and lead(IV) acetate
表征谱图
-
氢谱1HNMR
-
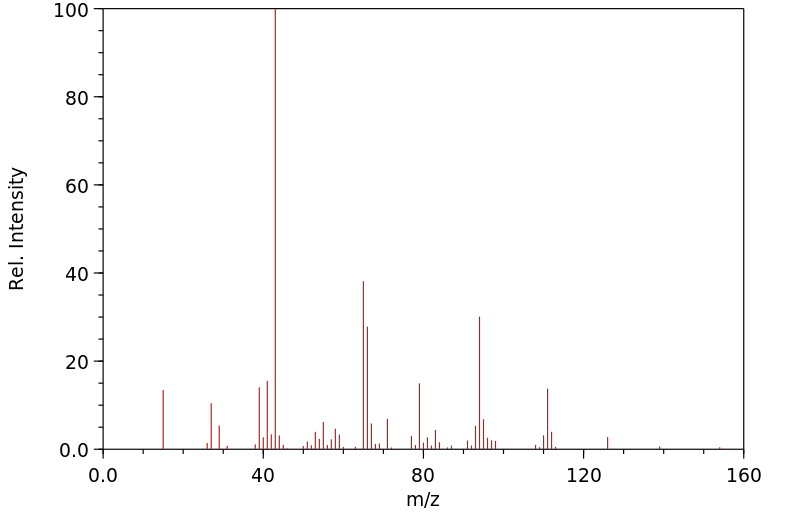
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸