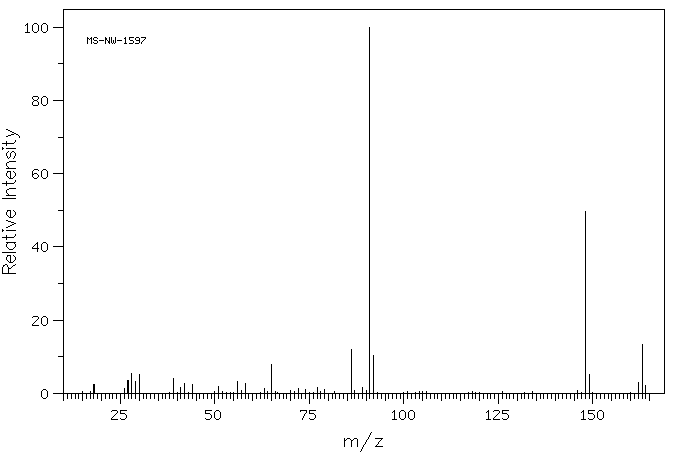
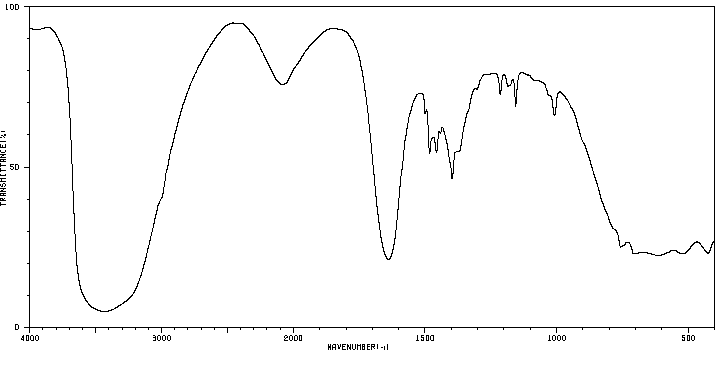
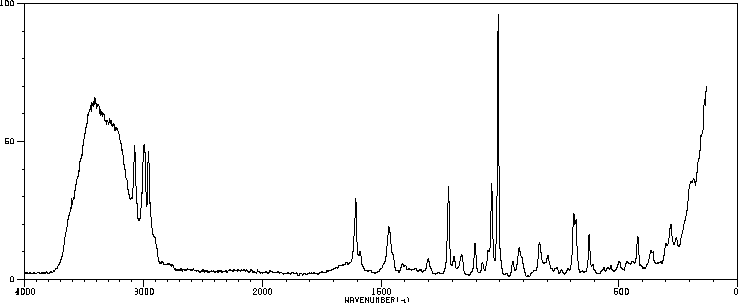
苄基三乙基氢氧化铵 | 1836-42-6
中文名称
苄基三乙基氢氧化铵
中文别名
氢氧化苄基三乙基铵;苄基四乙基氢氧化铵;三乙基苄基氫氧化胺
英文名称
benzyltriethylammonium hydroxide
英文别名
triethylbenzylammonium hydroxide;benzyl(triethyl)azanium;hydroxide
CAS
1836-42-6
化学式
C13H22N*HO
mdl
——
分子量
209.332
InChiKey
FKPSBYZGRQJIMO-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:0,92 g/cm3
-
闪点:26°C
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.89
-
重原子数:15
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.54
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:3
-
安全说明:S16,S26,S36/37/39,S45
-
危险类别码:R34,R10,R23/25
-
RTECS号:BS9625000
-
海关编码:2923900090
-
包装等级:II
-
危险类别:3
-
危险品运输编号:2924
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
Benzyltriethylammonium Hydroxide (10% in Water) Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Benzyltriethylammonium Hydroxide (10% in Water)
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 1C
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement Causes severe skin burns and eye damage
Precautionary statements
[Prevention] Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Store locked up.
[Storage]
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Mixture
Component(s): Benzyltriethylammonium Hydroxide (10% in Water)
....
Percent:
CAS Number: 1836-42-6
Chemical Formula: C13H23NO
Benzyltriethylammonium Hydroxide (10% in
Water)
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapor or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Benzyltriethylammonium Hydroxide (10% in
Water)
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
clear
Form:
Color: Colorless - Almost colorless
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
No data available
Lower:
Upper: No data available
Density: 1.02
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: scu-mus LDLo:160 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: BS9625000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. Observe all federal, state and local
regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
Benzyltriethylammonium Hydroxide (10% in
Water)
Section 14. TRANSPORT INFORMATION
UN-No: 3267
Proper shipping name: Corrosive liquid, basic, organic, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Benzyltriethylammonium Hydroxide (10% in Water)
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 1C
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement Causes severe skin burns and eye damage
Precautionary statements
[Prevention] Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Store locked up.
[Storage]
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Mixture
Component(s): Benzyltriethylammonium Hydroxide (10% in Water)
....
Percent:
CAS Number: 1836-42-6
Chemical Formula: C13H23NO
Benzyltriethylammonium Hydroxide (10% in
Water)
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (self-contained breathing apparatus). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapor or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Safety goggles. A face-shield, if the situation requires.
Eye protection:
Benzyltriethylammonium Hydroxide (10% in
Water)
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
clear
Form:
Color: Colorless - Almost colorless
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
No data available
Lower:
Upper: No data available
Density: 1.02
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: scu-mus LDLo:160 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: BS9625000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. Observe all federal, state and local
regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
Benzyltriethylammonium Hydroxide (10% in
Water)
Section 14. TRANSPORT INFORMATION
UN-No: 3267
Proper shipping name: Corrosive liquid, basic, organic, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:9-aminoalkylfluorenes摘要:含有9-氨基烷基取代基的萘类化合物是有用的抗心律失常药物。提供了含有这类化合物的药物配方,以及治疗方法。公开号:US04658061A1
-
作为产物:描述:参考文献:名称:Ladenburg; Struve, Chemische Berichte, 1877, vol. 10, p. 46摘要:DOI:
-
作为试剂:参考文献:名称:Yang, Zhi; Zhang, Shoufang, Journal of the Indian Chemical Society, 2002, vol. 79, # 8, p. 698 - 700摘要:DOI:
文献信息
-
Organic metal compound and process for preparing optically-active alcohols using the same申请人:Miki Takashi公开号:US20090062573A1公开(公告)日:2009-03-05The present invention provides an asymmetric reduction catalyst effective in preparing optically-active alcohol compounds having various functional groups, and a process for preparing optically-active alcohol compounds using said asymmetric reduction catalyst. The organic metal compound of the present invention is represented by the following general formula (1): wherein R 1 and R 2 may be mutually identical or different, and are an alkyl group, a phenyl group, a naphthyl group, a cycloalkyl group, or an alicyclic ring formed by binding R 1 and R 2 , which may have a substituent; R 3 is a hydrogen atom or an alkyl group; Cp is a cyclopentadienyl group, which may have a substituent, bound to M 1 via a π bond; X 1 is a halogen atom or a hydrido group; M 1 is rhodium or iridium; and * denotes asymmetric carbon.
-
Process for producing acrylic acid derivative申请人:Nippon Soda Co. Ltd公开号:US20040152894A1公开(公告)日:2004-08-05Processes for producing a compound represented by the formula (1), which includes an acrylic acid derivative and is useful as an agricultural chemical or medicine. One of the processes comprises the step of formulating a compound (3) and converting the OH of the resultant compound (2) into OR″. The first step comprises reacting a formic or orthoformic ester in the presence of a Lewis acid and a base. The second step comprises reacting the compound with R″OH or with R″OH and CH(OR″) 3 under acidic conditions or using a phase-transfer catalyst in a two-phase system and regulating the base and the concentration thereof to stereoselectively synthesize the target compound. In another, process, the compound is efficiently produced without isolating the compound. The compound can also be produced without the compound (2). 1
-
PROCESS FOR PREPARING DIARYL OXALATE申请人:Tanaka Shuji公开号:US20120296063A1公开(公告)日:2012-11-22Disclosed is a process for preparing a diaryl oxalate which comprises the step of transesterifying a dialkyl oxalate or/and an alkylaryl oxalate with an aryl alcohol in the presence of a tetra(aryloxy)titanium as a catalyst, wherein the tetra(aryloxy)titanium is fed into a reaction system of the transesterification as an aryl alcohol solution of the tetra(aryloxy)titanium which is prepared by reacting a tetraalkoxy titanium and an excess amount of the aryl alcohol and removing a by-producing alkyl alcohol.
-
ASYMMETRIC CATALYST AND PROCESS FOR PREPARING OPTICALLY ACTIVE ALCOHOLS USING THE SAME申请人:Watanabe Masahito公开号:US20100261924A1公开(公告)日:2010-10-14The present invention provides an organic metal compound, a ligand, an asymmetric catalyst, and a process for preparing optically-active alcohols using the asymmetric catalyst. The organic metal compound of the present invention is expressed by the following general formula (1): wherein in general formula (1), R 1 and R 2 are a mutually identical or mutually different, unsubstituted or substituted alkyl group, aryl group, cycloalkyl group, or R 1 and R 2 are bound to form an alicyclic ring, R 3 is a hydrogen atom or an alkyl group, R 4 is a branched alkyl group or an alkyl group that does or does not form a ring by itself, or an unsubstituted or substituted aryl group, or an unsubstituted or substituted heterocyclic group, Ar is an unsubstituted or substituted cyclopentadienyl group that is bound to M via a π bond, or an unsubstituted or substituted benzene, X is a hydride group or an anionic group, M is ruthenium, rhodium or iridium, L is a solvent molecule or a water molecule, l is 1 or 2, m is an integer from 0 to 2, n is 0 or 1, and when n is 0, X does not exist, and * represents asymmetric carbon, wherein R 4 is not a camphor group, a camphor derivative group, an isopropyl group or a phenyl group whenever R 1 and R 2 are both a phenyl group.本发明提供一种有机金属化合物、配体、不对称催化剂以及利用该不对称催化剂制备光学活性醇的方法。本发明的有机金属化合物由以下通用式(1)表示: 在通用式(1)中,R1和R2是相互相同或相互不同的未取代或取代的烷基基团、芳基团、环烷基团,或R1和R2结合形成脂环,R3是氢原子或烷基基团,R4是支链烷基基团或自身形成环或不形成环的烷基基团,或未取代或取代的芳基团,或未取代或取代的杂环基团,Ar是通过π键与M结合的未取代或取代的环戊二烯基团,或未取代或取代的苯,X是氢化物基团或阴离子基团,M是钌、铑或铱,L是溶剂分子或水分子,l为1或2,m为0到2的整数,n为0或1,当n为0时,X不存在,*代表不对称碳,其中当R1和R2均为苯基团时,R4不是藿香基团、藿香衍生基团、异丙基团或苯基团。
-
[EN] SYNTHESIS OF PYRAZOLES<br/>[FR] SYNTHÈSE DE PYRAZOLES申请人:WYETH CORP公开号:WO2009091832A1公开(公告)日:2009-07-23The present invention provides methods of making HCl salts, and methods of using them to modulate alpha7 nicotinic acetylcholine receptors and/or to treat any of a variety of disorders, diseases, and conditions. Provided compounds can affect, among other things, neurological, psychiatric and/or inflammatory system.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫