苄基正丁基醚 | 588-67-0
中文名称
苄基正丁基醚
中文别名
苄基丁基醚;苄丁醚;丁基苄基醚;1-苄氧丁烷
英文名称
benzyl 1-butyl ether
英文别名
benzyl butyl ether;(butoxymethyl)benzene;butoxymethylbenzene
CAS
588-67-0
化学式
C11H16O
mdl
MFCD00039954
分子量
164.247
InChiKey
MAYUYFCAPVDYBQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:111-112°C 23mm
-
密度:0,92 g/cm3
-
闪点:111-112°C/23mm
-
溶解度:Insoluble in water, soluble in alcohol and oils.
-
LogP:3.25
-
物理描述:Colourless liquid with a sweet, floral, somewhat pungent, aroma
-
折光率:1.480-1.485
-
保留指数:1238
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:12
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.454
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S24/25
-
海关编码:2909309090
-
储存条件:室温且干燥
SDS
苄基丁基醚 修改号码:5
模块 1. 化学品
产品名称: Benzyl Butyl Ether
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苄基丁基醚
百分比: >95.0%(GC)
CAS编码: 588-67-0
分子式: C11H16O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
苄基丁基醚 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-微浅黄色
气味: 无味
pH: 无数据资料
熔点: 无资料
沸点/沸程 220 °C
闪点: 91°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.92
苄基丁基醚 修改号码:5
模块 9. 理化特性
溶解度:
[水] 不溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
苄基丁基醚 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Benzyl Butyl Ether
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 警告
危险描述 可燃液体
防范说明
[预防] 远离明火/热表面。
穿戴防护手套/护目镜/防护面具。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 苄基丁基醚
百分比: >95.0%(GC)
CAS编码: 588-67-0
分子式: C11H16O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
苄基丁基醚 修改号码:5
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-微浅黄色
气味: 无味
pH: 无数据资料
熔点: 无资料
沸点/沸程 220 °C
闪点: 91°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.92
苄基丁基醚 修改号码:5
模块 9. 理化特性
溶解度:
[水] 不溶于
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
苄基丁基醚 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-苄氧基-1-丁醇 4-Benzyloxybutanol 4541-14-4 C11H16O2 180.247 —— (4-iodobutoxymethyl)benzene 50873-94-4 C11H15IO 290.144 —— 4-benzyloxy-2-butanol 4799-69-3 C11H16O2 180.247 4-苄氧基-2-丁酮 4-(benzyloxy)-2-butanone 6278-91-7 C11H14O2 178.231 —— ((3-bromobutoxy)methyl)benzene —— C11H15BrO 243.143 [(3-丁烯-1-基氧基)甲基]苯 4-benzyloxybut-1-ene 70388-33-9 C11H14O 162.232 —— ((but-3-yn-1-yloxy)methyl)benzene 22273-77-4 C11H12O 160.216 顺-1,4-二苄氧基-2-丁烯 (Z)-1,4-dibenzyloxy-2-butene 68972-96-3 C18H20O2 268.356 苯甲酸丁酯 Butyl benzoate 136-60-7 C11H14O2 178.231 —— benzaldehyde di-n-butylacetal 5395-08-4 C15H24O2 236.354 乙酰丙酮苄酯 benzyl acetoacetate 5396-89-4 C11H12O3 192.214 二苄醚 Benzyl ether 103-50-4 C14H14O 198.265 苯甲醇 benzyl alcohol 100-51-6 C7H8O 108.14 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(2-(苄氧基)乙基)环氧乙烷 2-(2-(benzyloxy)ethyl)oxirane 94426-72-9 C11H14O2 178.231 乙酸苄酯 Benzyl acetate 140-11-4 C9H10O2 150.177 苯甲酸丁酯 Butyl benzoate 136-60-7 C11H14O2 178.231 [丁氧基(苯基)甲基]苯 n-butyl diphenylmethyl ether 7495-83-2 C17H20O 240.345 苯甲醇 benzyl alcohol 100-51-6 C7H8O 108.14
反应信息
-
作为反应物:参考文献:名称:Cyclohexanones by Rh-Mediated Intramolecular C–H Insertion摘要:Some long chain alpha-aryl alpha-diazo ketones under Rh catalysis cyclize efficiently to the corresponding cyclohexanones. This is in marked contrast to the cyclizations of alpha-diazo beta-ketoesters, which consistently deliver cyclopentanone products.DOI:10.1021/jo4014996
-
作为产物:描述:顺-1,4-二苄氧基-2-丁烯 在 1-甲基吡咯烷 、 lithium aluminium tetrahydride 、 二氯二茂锆 作用下, 以 2-甲基四氢呋喃 为溶剂, 反应 20.0h, 以97%的产率得到苄基正丁基醚参考文献:名称:锆催化加氢金属化/β消除方法可还原性的CO,CN和CS裂解。摘要:据报道锆对Csp3和Csp2碳-杂原子键的催化还原裂解,利用链状烯烃官能团作为无痕导向基团。该反应已在CO,CN和CS键上成功证明,并建议通过原位形成的氢化锆催化剂的加氢锆/β-杂原子消除顺序进行。催化剂的位置异构化还能够裂解均烯丙基醚并除去末端的烯丙基和炔丙基。DOI:10.1021/acs.orglett.9b02572
-
作为试剂:参考文献:名称:[EN] RADIOIODINATED PHOSPHOLIPID ETHER ANALOGS AND METHODS OF USING THE SAME
[FR] ANALOGUES RADIO-IODES D'ETHERS PHOSPHOLIPIDIQUES ET LEURS PROCEDES D'UTILISATION摘要:(中) 描述了改进的放射性碘化磷脂醚类似物,它们表现出显著的肿瘤亲和性和比较短链类似物更长的血浆半衰期,提供了更优越的肿瘤病变成像和可视化以及针对肿瘤特异性细胞毒性癌症治疗。公开号:WO1998024480A1
文献信息
-
TAU-PROTEIN TARGETING PROTACS AND ASSOCIATED METHODS OF USE申请人:Arvinas, Inc.公开号:US20180125821A1公开(公告)日:2018-05-10The present disclosure relates to bifunctional compounds, which find utility as modulators of tau protein. In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a VHL or cereblon ligand which binds to the E3 ubiquitin ligase and on the other end a moiety which binds tau protein, such that tau protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of tau. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of tau protein. Diseases or disorders that result from aggregation or accumulation of tau protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为tau蛋白的调节剂具有实用性。具体而言,本公开涉及含有一端结合到E3泛素连接酶的VHL或cereblon配体,另一端结合到tau蛋白的双功能化合物,使得tau蛋白与泛素连接酶靠近,以实现tau蛋白的降解(和抑制)。本公开展示了与tau蛋白降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由tau蛋白聚集或积累导致的疾病或紊乱。
-
Direct and efficient synthesis of unsymmetrical ethers from alcohols catalyzed by Fe(HSO4)3 under solvent‐free conditions作者:Bashir Nazari Moghadam、Batool Akhlaghinia、Soodabeh RezazadehDOI:10.1007/s11164-015-2098-y日期:2016.2Highly efficient Fe(HSO4)3 catalyzed etherification of primary, secondary and tertiary benzylic alcohols with primary and secondary aliphatic alcohols is reported. The reaction affords unsymmetrical benzyl ethers in good to excellent yields under solvent-free conditions.报道了伯,仲和叔苄醇与伯和仲脂族醇的高效Fe(HSO 4)3催化醚化。该反应在无溶剂条件下以良好或优异的产率提供不对称的苄基醚。
-
一种卤化物氢解的方法
-
The Guanidine-Promoted Direct Synthesis of Open-Chained Carbonates作者:Yuhan Shang、Mai Zheng、Haibo Zhang、Xiaohai ZhouDOI:10.1071/ch18623日期:——
In order to reduce CO2 accumulation in the atmosphere, chemical fixation methodologies were developed and proved to be promising. In general, CO2 was turned into cyclic carbonates by cycloaddition with epoxides. However, the cyclic carbonates need to be converted into open-chained carbonates by transesterification for industrial usage, which results in wasted energy and materials. Herein, we report a process catalyzed by tetramethylguanidine (TMG) to afford linear carbonates directly. This process is greener and shows potential for industrial applications.
-
Amination of ethers using chloramine-T hydrate and a copper(i) catalyst作者:David P. Albone、Stephen Challenger、Andrew M. Derrick、Shaun M. Fillery、Jacob L. Irwin、Christopher M. Parsons、Hiroya Takada、Paul C. Taylor、D. James WilsonDOI:10.1039/b410883c日期:——by ether oxygen atoms is facile with chloramine-T as nitrene source and copper(I) chloride in acetonitrile as catalyst. For cyclic ethers the hemiaminal products are generally stable and can be isolated pure. For acyclic ethers, the hemiaminal products, as expected, fragment with elimination of alcohol to yield imines. When activation of benzylic positions is remote through a conjugated system, stable
表征谱图
-
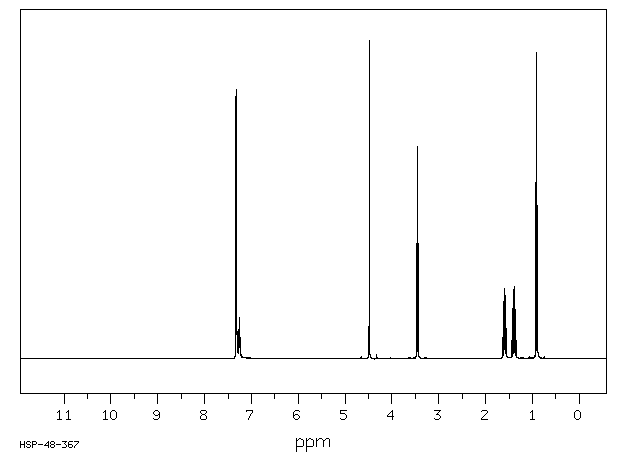
氢谱1HNMR
-
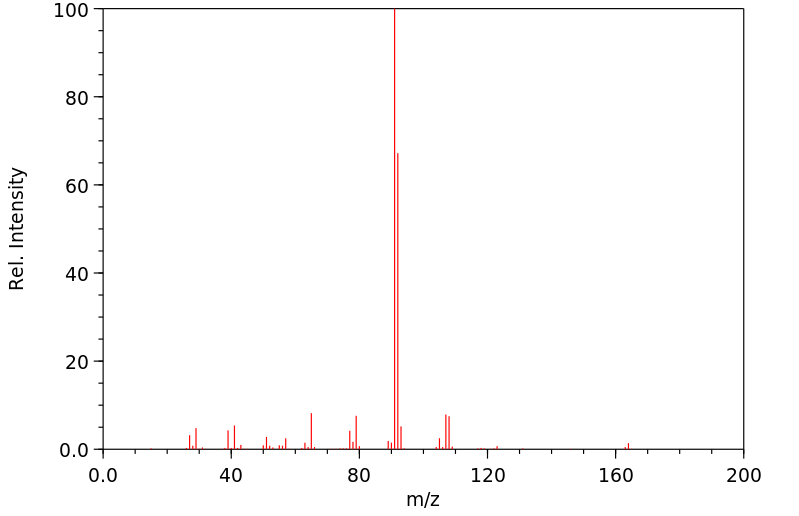
质谱MS
-
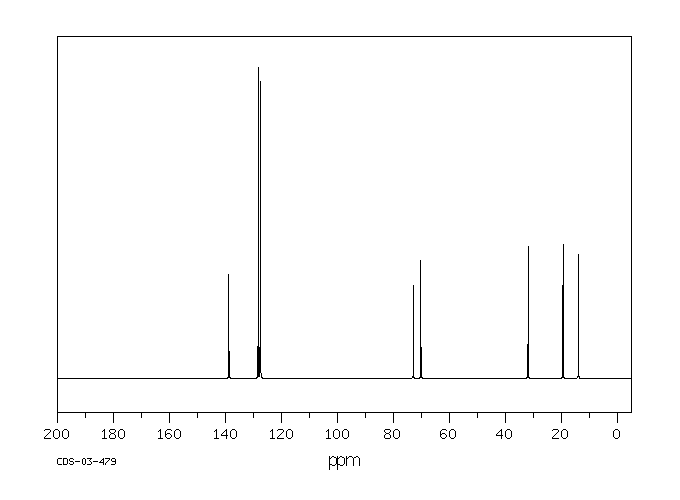
碳谱13CNMR
-
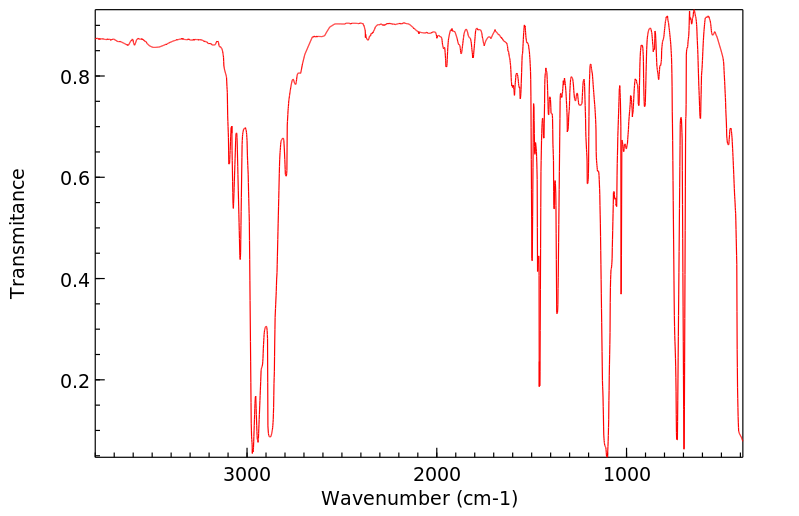
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫