6-氯黄酮 | 10420-73-2
中文名称
6-氯黄酮
中文别名
——
英文名称
6-chloroflavone
英文别名
6-chloro-2-phenyl-4H-chromen-4-one;6-chloro-2-phenylchromen-4-one
CAS
10420-73-2
化学式
C15H9ClO2
mdl
——
分子量
256.688
InChiKey
IFNDLWHUYFSXBK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:183-185 °C(lit.)
-
沸点:408.8±45.0 °C(Predicted)
-
密度:1.342±0.06 g/cm3(Predicted)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:18
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
储存条件:存放在阴凉干燥处即可。
SDS
| Name: | 6-Chloroflavone Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 10420-73-2 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 10420-73-2 | 6-Chloroflavone | 98 | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 10420-73-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 183 - 185 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density:
Molecular Formula: C15H9ClO2
Molecular Weight: 256.69
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 10420-73-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
6-Chloroflavone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 10420-73-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 10420-73-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 10420-73-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-氯色酮 6-chloro-4H-chromen-4-one 33533-99-2 C9H5ClO2 180.59 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,6-二氯-2-苯基-4H-色烯-4-酮 MRS 1088 13179-00-5 C15H8Cl2O2 291.133 —— 6-chloro-3-hydroxy-2-phenyl-4-oxo-4H-1-benzopyran 93986-79-9 C15H9ClO3 272.688 —— 4-Thio-6-chloro-flavon 59835-95-9 C15H9ClOS 272.755 —— diethyl (6-chloro-4-oxo-2-phenyl-4H-chromen-3-yl)phosphonate 1375533-50-8 C19H18ClO5P 392.776
反应信息
-
作为反应物:参考文献:名称:β-环糊精(β-CD)作为超分子催化剂合成异恶唑和硫嘧啶类化合物及其抗菌筛选摘要:背景:异恶唑和嘧啶是有趣的杂环分子,在许多天然产物合成药物中作为结构基序存在。异恶唑和硫代嘧啶具有抗糖尿病,细胞毒性结核抑制和抗惊厥活性。本文中,我们首次报道了在水介质中,β-环糊精作为可重复使用的催化剂,通过2-芳基色酮与盐酸羟胺和硫脲的开环反应,合成了异恶唑和硫代嘧啶。 方法:报道了2-芳基色酮与羟胺盐酸盐和硫脲分别在乙醇-水中超声作用下,β-环糊精催化合成异恶唑和硫代嘧啶衍生物。该反应在80°C进行,收率极佳,β-环糊精可回收再利用。评价了新合成的异恶唑和硫代嘧啶的抗菌活性。 结果:在继续我们对β-CD的研究和开发新方法的过程中,我们公开了一种新的合成异恶唑2a-n和硫代嘧啶3a-n的方法,该方法涉及2-芳基色酮与盐酸羟胺和硫脲的反应。 β-环糊精作为可循环利用催化剂在80oC的水中超声处理时,收率很高。由2-芳基色酮合成异恶唑2a-n和硫代嘧啶3a-n的一般方案描述于方案1。如此获得的产物的结构通过1DOI:10.2174/1570178614666161230124807
-
作为产物:参考文献:名称:通过分子内wittig反应的新型4H-chromen-4-ones合成摘要:由O-酰基(芳酰基)水杨酸的甲硅烷基酯与(三甲基甲硅烷基)亚甲基三苯基膦烷的甲硅烷基酯的反应顺序形成的酰基磷光烷在酯羰基上进行分子内的Wittig环化反应,得到良好或优异的4H-铬4--4-产量。DOI:10.1021/ol006518p
文献信息
-
Palladium-Catalyzed Carbonylation Reaction of Aryl Bromides with 2-Hydroxyacetophenones to Form Flavones作者:Xiao-Feng Wu、Helfried Neumann、Matthias BellerDOI:10.1002/chem.201202141日期:2012.10.1Flavone of the month: A general and efficient method for the palladium‐catalyzed carbonylative synthesis of flavones has been developed (see scheme). Starting from aryl bromides and 2‐hydroxyacetophenones, the corresponding flavones have been isolated in good yields.
-
Silver-catalyzed Double Decarboxylative Radical Alkynylation/Annulation of Arylpropiolic Acids with α-keto Acids: Access to Ynones and Flavones under Mild Conditions作者:Mengting Meng、Guofang Wang、Liangfeng Yang、Kai Cheng、Chenze QiDOI:10.1002/adsc.201701469日期:2018.3.20Ynones are privileged building blocks in various organic syntheses of heterocyclic derivatives due to their multifunctional nature, and flavones are an important class of natural products with a wide range of biological activities. We describe the catalytic double decarboxylative alkynylation of arylpropiolic acids with α‐keto acids. With Ag(I)/persulfate as the catalysis system, the valuable ynones
-
Identification and structure activity relationship of novel flavone derivatives that inhibit the production of nitric oxide and PGE 2 in LPS-induced RAW 264.7 cells作者:Ji-Young An、Hwi-Ho Lee、Ji-Sun Shin、Hyung-Seok Yoo、Jong Seon Park、Seung Hwan Son、Sang Won Kim、Jihyun Yu、Jun Lee、Kyung-Tae Lee、Nam-Jung KimDOI:10.1016/j.bmcl.2017.03.057日期:2017.6In an effort to identify novel anti-inflammatory compounds, a series of flavone derivatives were synthesized and biologically evaluated for their inhibitory effects on the production of nitric oxide (NO) and prostaglandin E2 (PGE2), representative pro-inflammatory mediators, in LPS-induced RAW 264.7 cells. Their structure-activity relationship was also investigated. In particular, we found that compound
-
One-Pot Allan-Robinson/Friedländer Route to Chromen-/Quinolin-4-ones through the Domino Acetylative Cyclisation of 2-Hydroxy-/2-Aminobenzaldehydes作者:Vijai K. Rai、Fooleswar Verma、Ganeshwar P. Sahu、Manorama Singh、Ankita RaiDOI:10.1002/ejoc.201701435日期:2018.1.31A domino reaction between 2‐hydroxy‐/2‐aminobenzaldehydes and α‐haloketones gives chromen‐4‐ones and quinolin‐4‐ones in good to excellent yields. This method represents a new extension of the Allan–Robinson and Friedländer reactions, and uses N‐heterocyclic‐carbene catalysis. This approach has the advantages of operational simplicity, ambient reaction conditions, and no by‐product formation.2-羟基/ 2-氨基苯甲醛与α-卤代酮之间的多米诺反应使chromen-4-ones和quinolin-4-ones的产率高到极好。该方法代表了Allan-Robinson和Friedländer反应的新扩展,并使用了N-杂环-卡宾催化。这种方法的优点是操作简单,环境反应条件好,并且不会形成副产物。
-
Transition-Metal-Free Intramolecular Ullmann-Type O-Arylation: Synthesis of Chromone Derivatives作者:Jie Zhao、Yufen Zhao、Hua FuDOI:10.1002/anie.201007302日期:2011.4.11Expect the upexpected: A transition‐metal‐free approach to access chromone derivatives has been developed. The intramolecular O‐arylation of substituted 1‐(2‐haloaryl)‐propane‐1,3‐diones in DMF in the presence of K2CO3 gave the corresponding target products in good to excellent yields (see scheme; DMF=N,N′‐dimethylformamide).
表征谱图
-
氢谱1HNMR
-
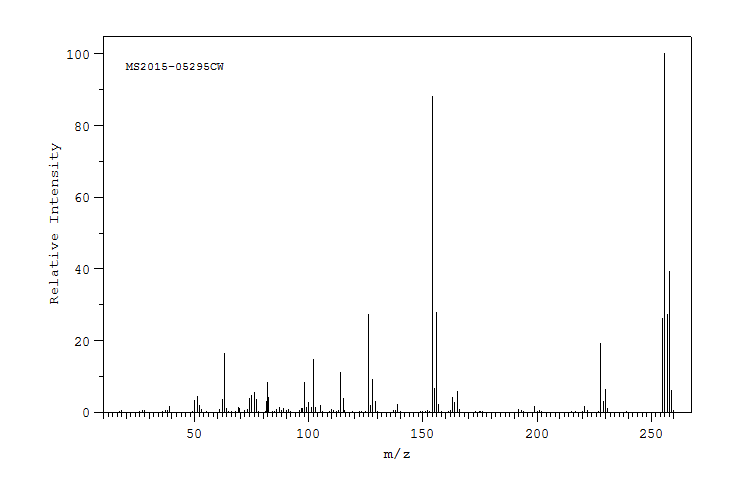
质谱MS
-
碳谱13CNMR
-
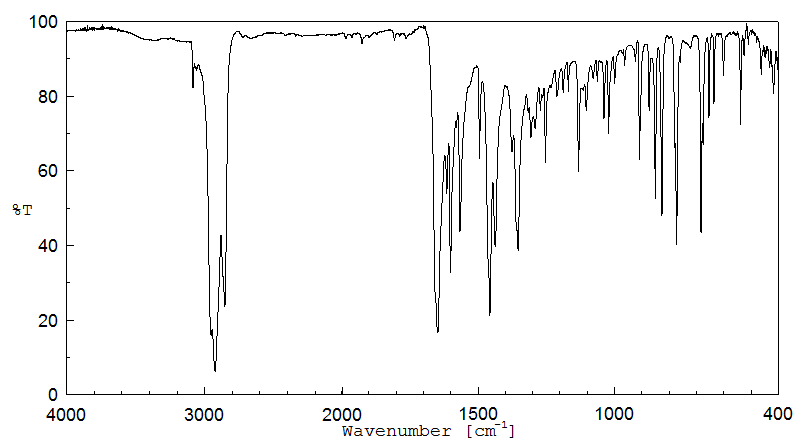
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷