9-氯代菲 | 947-72-8
中文名称
9-氯代菲
中文别名
9-氯菲
英文名称
9-chlorophenanthrene
英文别名
——
CAS
947-72-8
化学式
C14H9Cl
mdl
MFCD00037142
分子量
212.678
InChiKey
CJWHZQNUDAJJSB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:46-50 °C
-
沸点:275.35°C (rough estimate)
-
密度:1.1779 (rough estimate)
-
溶解度:氯仿(微溶)、乙酸乙酯(微溶)
-
保留指数:2012.7
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
海关编码:2903999090
SDS
| Name: | 9-Chlorophenanthrene 98% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 947-72-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 947-72-8 | 9-Chlorophenanthrene, 98% | 98% | 213-430-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Use with adequate ventilation. Avoid breathing dust, vapor, mist, or gas.
Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Good general ventilation should be sufficient to control airborne levels.
Exposure Limits CAS# 947-72-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: slightly yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 47 - 49 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H9Cl
Molecular Weight: 212.68
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 947-72-8: SF7325050 LD50/LC50:
Not available.
Carcinogenicity:
9-Chlorophenanthrene, 98% - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 947-72-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 947-72-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 947-72-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Sandqvist; Hagelin, Chemische Berichte, 1918, vol. 51, p. 1520摘要:DOI:
-
作为产物:描述:参考文献:名称:表面光化学:在氧化铝上用氯化铁对芳烃进行光氯化摘要:在氯化铁的存在下辐照吸附在氧化铝上的多种芳香烃会导致单氯化。DOI:10.1016/s0040-4039(01)92444-x
文献信息
-
Photoreduction of Pt(IV) Halo-Hydroxo Complexes: Possible Hypohalous Acid Elimination作者:Lasantha A. Wickramasinghe、Paul R. SharpDOI:10.1021/ic402358s日期:2014.2.3detected in photolyzed benzene solutions. Photolysis of 3 or 6 in the presence of 2,3-dimethyl-2-butene (TME) yields the chlorohydrin (2-chloro-2,3-dimethyl-3-butanol), 3-chloro-2,3-dimethyl-1-butene, and acetone, all expected products from HOCl trapping, but additional oxidation products are also observed. Photolysis of mixed chloro-bromo complex 7 with TME yields the bromohydrin (2-bromo-2,3-dimethyl-3-butanol)将浓缩的过氧化氢加至反式Pt(PEt 3)2 Cl(R)[ 1(R = 9-菲基),2(R = 4-三氟甲基苯基)]可以生成反式Pt(PEt 3)2( Cl)(OOH)(OH)(R)[ 5(R = 9-菲基),4(R = 4-三氟甲基苯基)],其中氢过氧配体反式为R。络合物5不稳定并与溶剂CH 2反应CL 2,得到的反式,顺式-Pt(PET 3)2(Cl)的2(OH)(9-菲基)(3)。用HCl处理4可获得类似的反式-顺式-Pt(PEt 3)2(Cl)2(OH)(4-三氟甲基苯基)(6)和HBr产生反式-Pt(PEt 3)2(Br)(Cl)( OH)(4-三氟甲基苯基)(7),其中Br和4-三氟甲基苯基配体是反式的。3或6在313或380 nm处发生光解会导致反式Pt(PEt 3)2 Cl(R)(1或2)。未检测到预期的副产物HOCl,但显示出真实的HOCl溶液在反应条件下会分解。在光解苯溶液中检测到氯苯和其他可将PPh
-
Photoreduction of Pt(IV) Chloro Complexes: Substrate Chlorination by a Triplet Excited State作者:Tharushi A. Perera、Mehdi Masjedi、Paul R. SharpDOI:10.1021/ic5009413日期:2014.7.21Except for 2 (R = Ph, 4-trifluoromethylphenyl), all of the Pt(IV) complexes are photosensitive to UV light and undergo net halogen reductive elimination to give Pt(II) products, trans-Pt(PEt3)2(R)(X) (X = Cl, Br). Chlorine trapping experiments with alkenes indicate a reductive-elimination mechanism that does not involve molecular chlorine and is sensitive to steric effects at the Pt center. DFT calculations通过氯化三氯甲烷制备反式-Pt(PEt 3)2(Cl)3(R)2(R = Cl,Ph,9-菲基,2-三氟甲基苯基,4-三氟甲基苯基,3- perenylenyl )的Pt(IV)配合物。 Pt(II)与-Cl 2(g)或PhICl 2的反式-Pt(PEt 3)2(R)(Cl)1络合物。混合的溴-氯配合物反式,反式-Pt(PEt 3)2(Cl)2(Br)(R)(R = 9-菲基,4-三氟甲基苯基),反式,顺式-Pt(PEt 3)2(Cl)2(Br)(4-三氟甲基苯基),反式,反式-Pt(PEt 3)2(Br)2(Cl)(R)(R = 9-菲基)和反式,顺式-Pt(PEt 3)2( BR)2(Cl)的(4-三氟甲基苯基)通过卤化物交换或通过氧化加成的Br获得2至1或Cl 2至反式-Pt(PET 3)2(R)(BR)。除2(R = Ph,4-三氟甲基苯基)外,所有Pt(IV)配合物均对紫外光敏感
-
Microwave-assisted synthesis of substituted phenanthrenes, anthracenes, acenaphthenes, and fluorenes作者:Shiuh-Chuan Chan、Jing-Pei Jang、Yie-Jia CherngDOI:10.1016/j.tet.2009.01.029日期:2009.3Rapid coupling reactions of polycyclic aromatic halides with various N-, S-, and Se-nucleophiles under focused microwave irradiation are described. Using this method, the desired products are obtained with good to excellent yields in a short reaction time.描述了在聚焦微波辐射下多环芳族卤化物与各种N-,S-和Se-亲核试剂的快速偶联反应。使用这种方法,可以在短的反应时间内以良好或极好的收率获得所需的产物。
-
PROCESS FOR PRODUCING AROMATIC AMINES申请人:TAKASAGO INTERNATIONAL CORPORATION公开号:US20020035295A1公开(公告)日:2002-03-21The present invention provides an activator in arylamination using a palladium compound as a catalyst, which is superior to conventional phosphines in stability and performance. With the phosphine sulfide as an activator, an arylamination reaction achieves improved selectivity to produce a desired aromatic amine in an obviously increased yield as compared with a reaction using the corresponding phosphine compound. Moreover, the phosphine sulfide of the invention is impervious to oxidation and exists stably in air and therefore sufficiently withstands use on an industrial scale.
-
Base-Mediated Intermolecular sp<sup>2</sup> C−H Bond Arylation via Benzyne Intermediates作者:Thanh Truong、Olafs DaugulisDOI:10.1021/ja200184b日期:2011.3.30A transition-metal-free method for arylation of heterocycle and arene carbon-hydrogen bonds by aryl chlorides and fluorides has been developed. The reactions proceed via aryne intermediates and are highly regioselective with respect to the C-H bond coupling component.
表征谱图
-
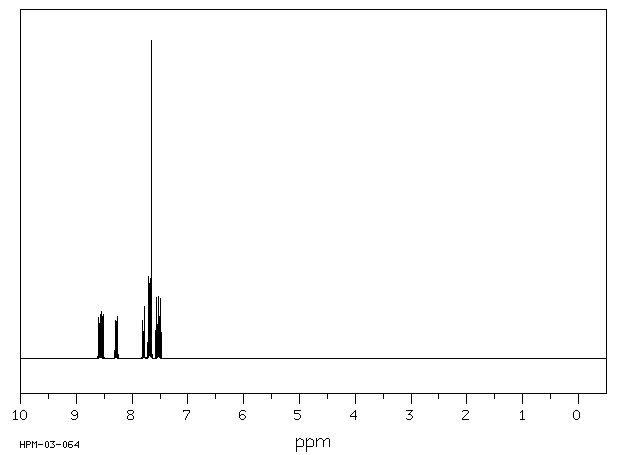
氢谱1HNMR
-
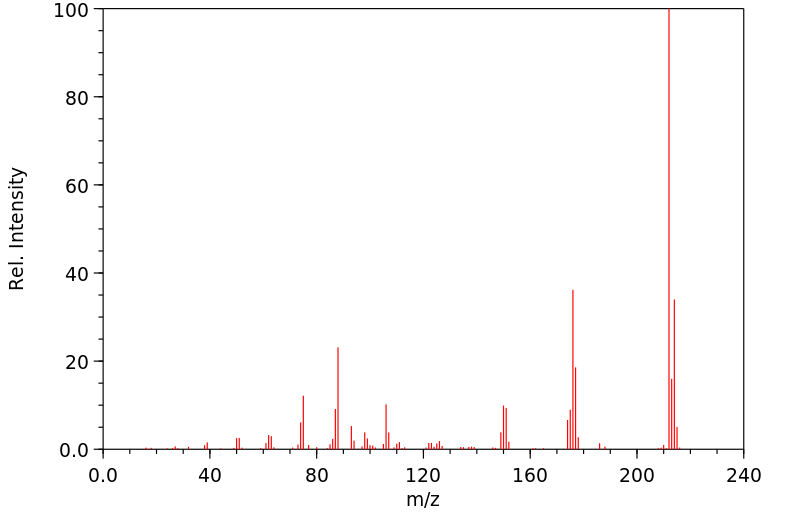
质谱MS
-
碳谱13CNMR
-
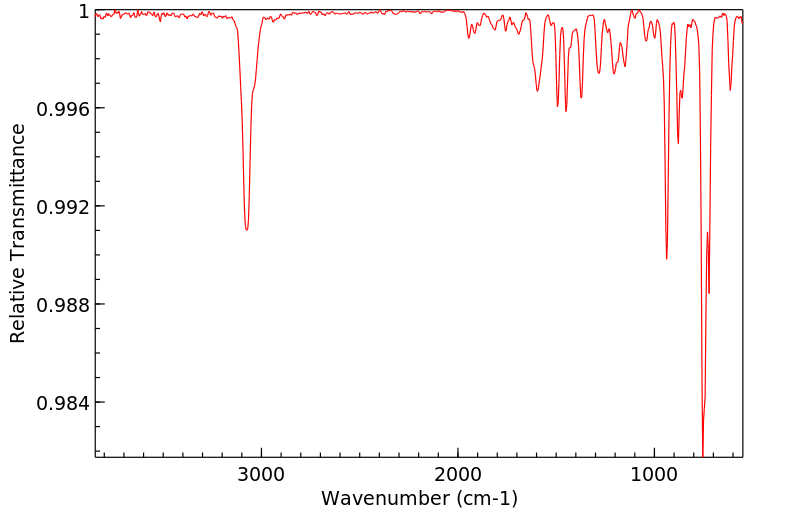
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2,2'',3,3''-四氢-6,6''-二-9-菲基-1,1''-螺双[1H-茚]-7,7''-二醇
(6,6)-苯基-C61己酸甲酯
高雌二醇
马兜铃酸钠
马兜铃酸盐
马兜铃酸C
马兜铃酸B
马兜铃酸(1:1MIXTUREOFARISTOLOCHICACIDIANDARISTOLOCHICACIDII)
马兜铃酸 Ia
马兜铃酸 IVa
马兜铃酸
颜料黑32
颜料红179
颜料红178
颜料红149
颜料红123
顺式-菲-1,2-二醇-3,4-环氧化物
顺式-苯并(a)屈-11,12-二醇-13,14-环氧化物
雷公藤酚A
镁二(1,4,5,6,7,16,17,18,19,19,20,20-十二氯六环[14.2.1.14,7.02,15.03,8.09,14]二十-5,9,11,13,17-五烯-11-磺酸酯)
钩大青酮
钩大青酮
钙(2+)12-羟基十八烷酸酯
酒石酸布托诺啡
那布扶林
还原红32
足球烯
贝那他汀B
贝母兰素
萘并[2,3-b]荧蒽
萘并[2,1-e][1]苯并二硫杂环戊烷
萘并[2,1-C:7,8-C']二菲
萘并[1,2-e][2]苯并呋喃-1,3-二酮
萘并[1,2-b]屈
萘并[1,2-a]蒽
萘并[1,2-B]菲-6-醇
萘二(六氯环戊二烯)加合物
萘,8-溴-1,2,3-三(1,1-二甲基乙基)-6-甲基-
菲醌单缩氨基硫脲
菲醌
菲并[9,10]呋喃
菲并[9,10-e]醋菲烯
菲并[4,5-bcd]噻吩
菲并[4,5-bcd]呋喃-3-醇
菲并[4,3-d]-1,3-二噁唑-5-羧酸,10-羟基-9-甲氧基-6-硝基-
菲并[3,2-b]噻吩
菲并[2,1-d]噻唑
菲并[2'',1'',10'':4,5,6;7'',8'',9'':4',5',6']二异喹啉并[2,1-a:2',1'-a']二萘嵌间二氮杂苯-8,13-二酮
菲并(3,4-b)噻吩
菲并(1,2-b)噻吩