DL-蛋氨酸乙酯盐酸盐 | 6297-53-6
中文名称
DL-蛋氨酸乙酯盐酸盐
中文别名
DL-蛋氨酸乙酯盐酸盐,98+
英文名称
methionine ethyl ester hydrochloride
英文别名
Methionine ethylester *HCl;(1-Ethoxy-4-methylsulfanyl-1-oxobutan-2-yl)azanium;chloride;(1-ethoxy-4-methylsulfanyl-1-oxobutan-2-yl)azanium;chloride
CAS
6297-53-6
化学式
C7H15NO2S*ClH
mdl
——
分子量
213.729
InChiKey
KPWCQEUBJAIORR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:72-75°C
-
稳定性/保质期:
在常温常压下,该物质稳定存在,表现为白色晶体状粉末。
计算性质
-
辛醇/水分配系数(LogP):1.05
-
重原子数:12
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:77.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
海关编码:2930909090
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P362,P403+P233,P501
-
危险性描述:H315,H319,H335
-
储存条件:0-5℃条件下应避光、存放在阴凉干燥处并密封保存。
SDS
反应信息
-
作为反应物:描述:DL-蛋氨酸乙酯盐酸盐 在 N,N-二异丙基乙胺 作用下, 以 四氢呋喃 为溶剂, 生成 2-[4-Chloro-6-(4-chloro-phenylamino)-[1,3,5]triazin-2-ylamino]-4-methylsulfanyl-butyric acid ethyl ester参考文献:名称:1,3,5-Triazine-Based Mass Spectral Tagging of One-Bead One-Compound Libraries摘要:A triazine-based mass encoding strategy that accommodates cleavable linker, isotopic labeling, and diversity receptor moieties is reported. The resulting triazine-based tags, which are coupled to bifunctionalized TentaGel resin in a one-pot transformation, enable the construction of a 1-oxa-2,8-diazaspiro[4.5]dec-2-ene-7-carboxamide library and facilitate decoding by equalizing the ionization potential of the liberated tags in single bead MALDI-TOF experiments as well as balancing the reactivity of the starting tags in the resin coupling step.DOI:10.1021/ol062466d
-
作为产物:描述:参考文献:名称:Anthranilic acid based CCK1 receptor antagonists: Blocking the receptor with the same ‘words’ of the endogenous ligand摘要:The anthranilic acid diamides represent the more recent class of nonpeptide CCK1 receptor antagonists. This class is characterized by the presence of anthranilic acid, used as a molecular scaffold, and two pharmacophores selected from the C-terminal tetrapeptide of CCK. The lead compound coded VL-0395, endowed with sub-micromolar affinity towards CCK1 receptors, was characterized by the presence of Phe and 2-indole moiety at the C- and N-termini of anthranilic acid, respectively. Herein we describe the first step of the anthranilic acid C- terminal optimization using, instead of Phe, aminoacids belonging to the primary structure of CCK-8 and other not coded residues. Thus we demonstrate that the CCK1 receptor affinity depends on the nature of the aminoacidic side chain as well as that the free carboxy group of the alpha-aminoacids is crucial for the binding. The R enantiomers of the most active compounds represent the eutomers of this class of antagonists confirming thus the stereo preference of the receptor. Moreover this SAR study demonstrates that the receptor binding pocket, that host the aminoacidic side chain, results much more tolerant respect to that accommodating the indole ring. As a result, an appropriate variation of the aminoacidic side chain could provide a better CCK1 receptor affinity diorthosis. (C) 2009 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2009.02.012
文献信息
-
Microwave-Assisted Ruthenium-Catalysed <i>ortho</i> -C−H Functionalization of <i>N</i> -Benzoyl <i>α</i> -Amino Ester Derivatives作者:Nandini Sharma、Vijay Bahadur、Upendra K. Sharma、Debasmita Saha、Zhenghua Li、Yogesh Kumar、Jona Colaers、Brajendra K Singh、Erik V. Van der EyckenDOI:10.1002/adsc.201800458日期:2018.8.17A microwave‐assisted highly efficient intermolecular C−H functionalization sequence has been developed to access substituted isoquinolones using α‐amino acid esters as a directing group. This methodology enables a wide range of N‐benzoyl α‐amino ester derivatives to react via a Ru‐catalysed C−H bond activation sequence, to form isoquinolones with moderate to excellent yields. As an additional advantage
-
Cycloalkyl, lactam, lactone and related compounds, pharmaceutical compositions comprising same, and methods for inhibiting beta-amyloid peptide release and/or its synthesis by use of such compounds申请人:——公开号:US20020045747A1公开(公告)日:2002-04-18Disclosed are compounds which inhibit &bgr;-amyloid peptide release and/or its synthesis, and, accordingly, have utility in treating Alzheimer's disease. Also disclosed are pharmaceutical compositions comprising a compound which inhibits &bgr;-amyloid peptide release and/or its synthesis as well as methods for treating Alzheimer's disease both prophylactically and therapeutically with such pharmaceutical compositions.公开了抑制β-淀粉样肽释放和/或其合成的化合物,因此可用于治疗阿尔茨海默病。还公开了包含抑制β-淀粉样肽释放和/或其合成的化合物的药物组合物,以及使用这些药物组合物预防性和治疗性治疗阿尔茨海默病的方法。
-
Inhibitors of protein isoprenyl transferases申请人:University of Pittsburgh公开号:US20020193596A1公开(公告)日:2002-12-19Compounds having the formula 1 or a pharmaceutically acceptable salt thereof wherein R 1 is (a) hydrogen, (b) loweralkyl, (c) alkenyl, (d) alkoxy, (e) thioalkoxy, (f) halo, (g) haloalkyl, (h) aryl-L 2 —, and (i) heterocyclic-L 2 —; R 2 is selected from (a) 2 (b) —C(O)NH—CH(R 14 )—C(O)OR 15 , (c) 3 (d) —C(O)NH—CH(R 14 )—C(O)NHSO 2 R 16 (e) —C(O)NH—CH(R 14 )-tetrazolyl, (f) —C(O)NH-heterocyclic, and (g) —C(O)NH—CH(R 14 )—C(O)NR 17 R 18 ; R 3 is heterocyclic, aryl, substituted or unsubstituted cycloalkyl; R 4 is hydrogen, lower alkyl, haloalkyl, halogen, aryl, arylakyl, heterocyclic, or (heterocyclic)alkyl; L 1 is absent or is selected from (a) —L 4 —N(R 5 )—L 5 —, (b) —L 4 —O—L 5 —, (c) —L 4 —S(O) n —L 5 — (d) —L 4 -L 6 —C(W)—N(R 5 )—L 5 —, (e) —L 4 -L 6 —S(O) m —N(R 5 )—L 5 —, (f) —L 4 —N(R 5 )—C(W)—L 7 -L 5 —, (g) —L 4 —N(R 5 )—S(O) p —L 7 —L 5 —, (h) optionally substituted alkylene, (i) optionally substituted alkenylene, and (j) optionally substituted alkynylene are inhibitors of protein isoprenyl transferases. Also disclosed are protein isoprenyl transferase inhibiting compositions and a method of inhibiting protein isoprenyl transferases.具有以下公式的化合物或其药学上可接受的盐,其中R1为(a)氢,(b)较低的烷基,(c)烯基,(d)烷氧基,(e)硫代烷氧基,(f)卤素,(g)卤代烷基,(h)芳基-L2—,以及(i)杂环-L2—;R2从(a)中选择,(b) -C(O)NH-CH(R14)-C(O)OR15,(c)中选择,(d) -C(O)NH-CH(R14)-C(O)NHSO2R16,(e) -C(O)NH-CH(R14)-四唑基,(f) -C(O)NH-杂环,以及(g) -C(O)NH-CH(R14)-C(O)NR17R18;R3为杂环,芳基,取代或未取代的环烷基;R4为氢,较低烷基,卤代烷基,卤素,芳基,芳基烷基,杂环基,或(杂环)烷基;L1为空缺或从(a) -L4-N(R5)-L5-,(b) -L4-O-L5-,(c) -L4-S(O)n-L5-,(d) -L4-L6-C(W)-N(R5)-L5-,(e) -L4-L6-S(O)m-N(R5)-L5-,(f) -L4-N(R5)-C(W)-L7-L5-,(g) -L4-N(R5)-S(O)p-L7-L5-,(h)可选择取代的烷基,(i)可选择取代的烯基,以及(j)可选择取代的炔基是蛋白异戊二烯转移酶的抑制剂。还公开了蛋白异戊二烯转移酶抑制组合物和抑制蛋白异戊二烯转移酶的方法。
-
Heterocyclic compounds, pharmaceutical compositions comprising same, and methods for inhibiting &bgr;-amyloid peptide release and/or its synthesis by use of such compounds申请人:Athena Neurosciences, Inc.公开号:US06506782B1公开(公告)日:2003-01-14Disclosed are compounds which inhibit &bgr;-amyloid peptide release and/or its synthesis, and, accordingly, have utility in treating Alzheimer's disease. Also disclosed are pharmaceutical compositions comprising a compound which inhibits &bgr;-amyloid peptide release and/or its synthesis as well as methods for treating Alzheimer's disease both prophylactically and therapeutically with such pharmaceutical compositions.公开了抑制β-淀粉样肽释放和/或其合成的化合物,因此可用于治疗阿尔茨海默病。还公开了包含抑制β-淀粉样肽释放和/或其合成的化合物的药物组合物,以及使用这些药物组合物预防性和治疗性地治疗阿尔茨海默病的方法。
-
Compounds for inhibiting &bgr;-amyloid peptide release and/or its synthesis申请人:Elan Pharmaceuticals, Inc.公开号:US06211235B1公开(公告)日:2001-04-03Disclosed are compounds which inhibit &bgr;-amyloid peptide release and/or its synthesis, and, accordingly, have utility in treating Alzheimer's disease. Also disclosed are pharmaceutical compositions comprising a compound which inhibits &bgr;-amyloid peptide release and/or its synthesis.揭示了一种抑制β-淀粉样肽释放和/或其合成的化合物,因此在治疗阿尔茨海默病方面具有用途。还揭示了包含抑制β-淀粉样肽释放和/或其合成的化合物的药物组合物。
表征谱图
-
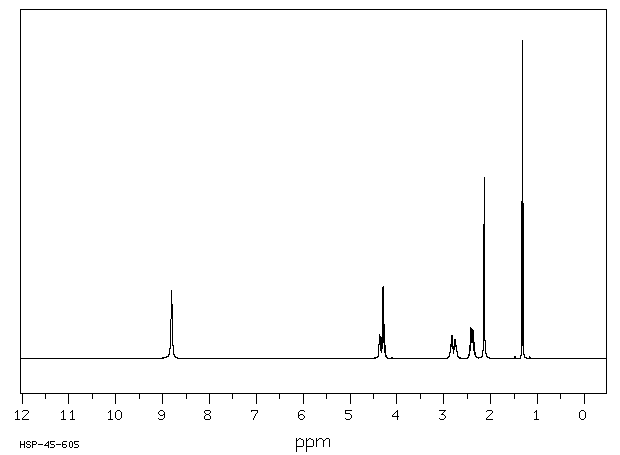
氢谱1HNMR
-
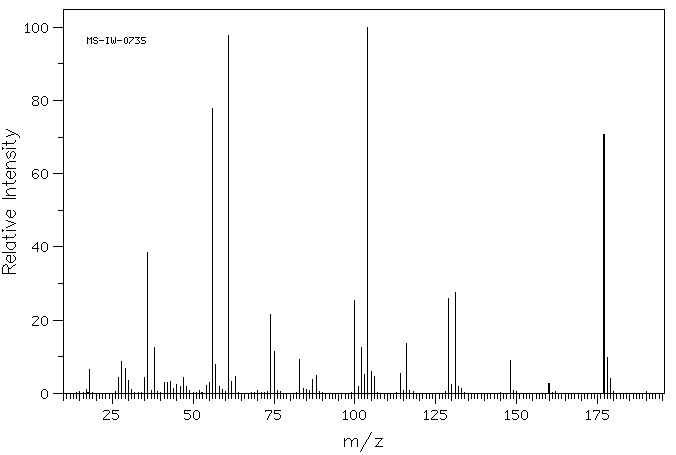
质谱MS
-
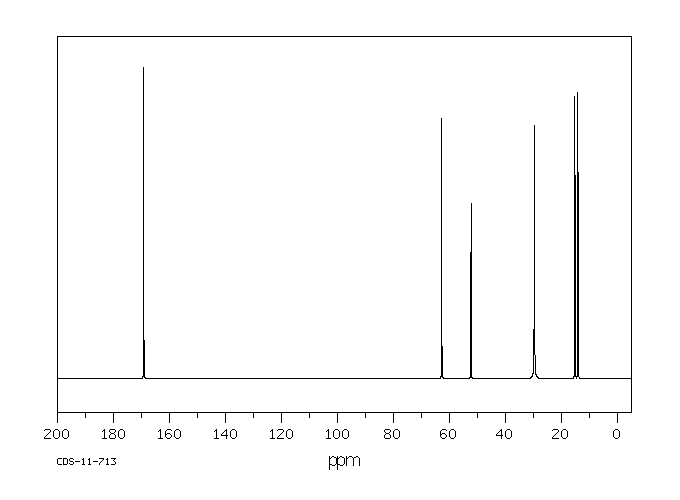
碳谱13CNMR
-
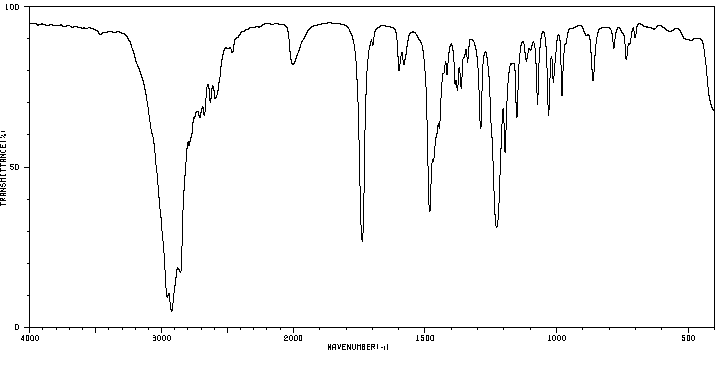
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸