L-氢化乳清酸 | 5988-19-2
中文名称
L-氢化乳清酸
中文别名
腺苷-5-磷酸(AMP);鸟苷磷酸二钠盐(GMP);核苷酸;核糖核酸(酵母)RNA;L-4,5-二氢乳清酸;(S)-2,6-二氧代六氢-4-嘧啶羧酸
英文名称
L-dihydroorotic acid
英文别名
L-4,5-dihydroorotic acid;(S)-2,6-dioxohexahydropyrimidine-4-carboxylic acid;(S)-dihydroorotic acid;L-dihydroortotic acid;L-hydroorotic acid;dihydroorotic acid;(4S)-2,6-dioxo-1,3-diazinane-4-carboxylic acid
CAS
5988-19-2
化学式
C5H6N2O4
mdl
MFCD00085339
分子量
158.114
InChiKey
UFIVEPVSAGBUSI-REOHCLBHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:254-255 °C (dec.)(lit.)
-
沸点:283.16°C (rough estimate)
-
密度:1.523
-
溶解度:DMSO(微溶)、水(微溶、加热、超声处理)
-
物理描述:Solid
-
碰撞截面:145.44 Ų [M-H]- [CCS Type: DT, Method: stepped-field]
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-1.5
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:95.5
-
氢给体数:3
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:NONH for all modes of transport
-
海关编码:2934999001
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:建议存储于0-5℃环境中,避光、阴凉且干燥的地方,并密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
L-Dihydroorotic acid
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P302+P352: IF ON SKIN: Wash with soap and water
P321: Specific treatment (see on this label)
P405: Store locked up
Section 3. Composition/information on ingredients.
L-Dihydroorotic acid
Ingredient name:
CAS number: 5988-19-2
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C5H6N2O4
Molecular weight: 158.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
L-Dihydroorotic acid
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P302+P352: IF ON SKIN: Wash with soap and water
P321: Specific treatment (see on this label)
P405: Store locked up
Section 3. Composition/information on ingredients.
L-Dihydroorotic acid
Ingredient name:
CAS number: 5988-19-2
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C5H6N2O4
Molecular weight: 158.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
性状
核苷酸为白色结晶性粉末。
生理作用核苷酸(nucleotides,NTs)是一类具有重要生物学功能的小分子,是生物大分子核酸(nucleic acid,NA),即DNA和RNA的前体物,参与了机体内几乎所有的生物化学反应。生物体既可以进行从头合成也可以利用细胞内代谢产物进行补救合成。同时,核苷酸还是人类饮食中的常见组成成分,它们可以被吸收并结合为机体组织的一部分。
由于膳食核苷酸及核酸在被消化、吸收以及体内代谢上有基本相同的代谢途径,生物学作用也十分相似,所以有理由推测,不论是口服核苷酸还是核酸,它们在体内发挥的营养作用是相似的。
应用调味剂与增味剂
母乳中含有尿苷酸(UMP)、胞苷酸(CMP)、腺苷酸(AMP)、鸟苷酸(GMP)、肌苷酸(IMP)等多种核苷酸,为提高婴儿的免疫调节功能和记忆力发挥着作用。在欧美、日本等国家生产的婴儿奶粉中均添加了微量核苷酸,也有添加RNA的例子。
医药原料
核苷酸作为医药用途,可抑制尿道发炎。
食品添加剂最大允许使用量与最大允许残留量标准- 添加剂中文名称:核苷酸
- 允许使用该种添加剂的食品中文名称:婴幼儿配方粉
- 添加剂功能:营养强化剂
- 最大允许使用量(g/kg):0.12-0.58g/kg(以核苷酸总量计)
L-Dihydroorotic acid (L-dihydroorotate) 是嘧啶代谢的中间产物,它是二氢乳清酸脱氢酶(线粒体)的底物。
用途
用作增鲜剂、调味剂,可用于家庭、餐饮业等。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-甲基-L-4,5-二氢乳清酸 (S)-3-methyldihydroorotic acid 103365-69-1 C6H8N2O4 172.141 (S)-六氢-2,6-二氧代-4-嘧啶甲酸苯甲酯 benzyl (S)-dihydroorotate 103300-84-1 C12H12N2O4 248.238 苄基(4S)-1-甲基-2,6-二氧代六氢-4-嘧啶羧酸酯 benzyl (S)-3-methyldihydroorotate 103300-85-2 C13H14N2O4 262.265
反应信息
-
作为反应物:参考文献:名称:Stereoselectivity in the enzymic oxidation and nonenzymic hydrogen-exchange reactions of dihydroorotate摘要:DOI:10.1021/ja00288a036
-
作为产物:参考文献:名称:Lieberman; Kornberg, 1953, vol. 12, p. 223,227摘要:DOI:
文献信息
-
[EN] FERROPTOSIS INHIBITORS–DIARYLAMINE PARA-ACETAMIDES<br/>[FR] INHIBITEURS DE FERROPTOSE-DIARYLAMINE PARA-ACÉTAMIDES申请人:SIRONAX LTD公开号:WO2021175200A1公开(公告)日:2021-09-10Provided are compounds that inhibit ferroptosis activity, or modulate or inhibit a disease associated with ferroptosis dysregulation, such as neuropathy, ischemia reperfusion injury, acute kidney failure and cancer, including corresponding sulfonamides, and pharmaceutically acceptable salts, hydrates and stereoisomers thereof. The compounds are employed in pharmaceutical compositions, and methods of making and use, including treating a person in need thereof with an effective amount of the compound or composition, and detecting a resultant improvement in the person's health or condition.
-
Structural, Electronic, and Electrostatic Determinants for Inhibitor Binding to Subsites S1 and S2 in SARS-CoV-2 Main Protease作者:Daniel W. Kneller、Hui Li、Stephanie Galanie、Gwyndalyn Phillips、Audrey Labbé、Kevin L. Weiss、Qiu Zhang、Mark A. Arnould、Austin Clyde、Heng Ma、Arvind Ramanathan、Colleen B. Jonsson、Martha S. Head、Leighton Coates、John M. Louis、Peter V. Bonnesen、Andrey KovalevskyDOI:10.1021/acs.jmedchem.1c01475日期:2021.12.9acute respiratory syndrome coronavirus 2 (SARS-CoV-2) proteins is crucial to battle coronavirus disease 2019 (COVID-19). SARS-CoV-2 main protease (Mpro) is an established drug target for the design of protease inhibitors. We performed a structure–activity relationship (SAR) study of noncovalent compounds that bind in the enzyme’s substrate-binding subsites S1 and S2, revealing structural, electronic开发针对严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 蛋白的小分子抗病毒药物对于对抗 2019 年冠状病毒病 (COVID-19) 至关重要。 SARS-CoV-2 主要蛋白酶 (M pro ) 是设计蛋白酶抑制剂的既定药物靶点。我们对与酶的底物结合亚位点 S1 和 S2 结合的非共价化合物进行了构效关系 (SAR) 研究,揭示了这些位点的结构、电子和静电决定因素。该研究以 M pro与 Mcule-5948770040(化合物1 )复合的 X 射线/中子结构为指导,其中质子化状态直接可视化。采用虚拟现实辅助结构分析和小分子构建来生成1的类似物。体外酶抑制测定和室温 X 射线结构证明了化学修饰对 M pro抑制的影响,表明(1)维持抑制剂 P1 基团的正确几何形状对于保留与质子化的 His163 的氢键至关重要; (2)优选带正电荷的接头; (3) 亚位点 S2 更喜欢体积较小的适度电负性基团。
-
Synthesis and central nervous system actions of thyrotropin-releasing hormone analog containing a dihydroorotic acid moiety作者:Mamoru Suzuki、Hiroshi Sugano、Kazuo Matsumoto、Michio Yamamura、Ryuichi IshidaDOI:10.1021/jm00170a014日期:1990.8A series of thyrotropin-releasing hormone (TRH) analogues in which the pyroglutamic acid residue was replaced by (S)-4,5-dihydroorotic acid (Dio-OH) and the related derivatives were prepared. Their central nervous system actions based on spontaneous locomotor activity, antagonistic effect on reserpine-induced hypothermia, and antagonistic effect on pentobarbital anesthesia were evaluated and the structure-activity
-
Practical syntheses of optically pure 1- and 3-substituted dihydroorotic acids.作者:Mamoru SUZUKI、Hiroshi MAEDA、Kazuhiko KONDO、Hiroshi SUGANO、Kazuo MATSUMOTODOI:10.1248/cpb.37.1764日期:——Various optically pure 3-substituted dihydroorotic acids (9, 12) were synthesized by the N-alkylation and Mitsunobu reaction of optically active benzyl or tert-butyl dihydroorotate (6, 10), followed by deprotection of the ester group.Moreover, optically pure 1-methyl (16, 17) and 1-ethyl (19) dihydroorotic acids were obtained by an optical resolution method.
-
Gonadotropin-releasing hormone receptor-targeted paclitaxel-degarelix conjugate: synthesis and <i>in vitro</i> evaluation作者:Chenhong Wang、Yongtao Ma、Siliang Feng、Keliang Liu、Ning ZhouDOI:10.1002/psc.2769日期:2015.7degarelix. The in vitro cytostatic effects of the conjugates were determined by a (3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium) (MTS) assay, and the 50% inhibitory concentration value of the conjugates on 3T3 mouse embryonic fibroblast cells were one order of magnitude higher than the 50% inhibitory concentration values of the conjugates on MCF‐7 human breast为了增加化学治疗剂的选择性,已经开发了受体介导的肿瘤靶向方法。这里,地加瑞克[的Ac- d -Nal- d -Cpa- d -PAL-SER-APH(大号-Hor) - d -Aph(CBM)-Leu-ILys-亲d -Ala-NH 2],一种促性腺激素释放激素拮抗剂,被用作紫杉醇(PTX)的靶向部分。合成了五种PTX-degarelix共轭物,其中PTX通过二硫键连接到degarelix序列中的不同位置。在人血清中测定的所有PTX–degarelix结合物的半衰期均大于10小时。荧光成像板读数器测定表明,缀合物LK‐MY‐9和LK‐MY‐10具有与地加瑞克相似的拮抗作用。在体外通过(3-(4,5-二甲基噻唑-2-基)-5-(3-羧基甲氧基苯基)-2-(4-磺基苯基)-2H-四唑鎓)(MTS)测定确定缀合物的细胞抑制作用结合物对3T3小鼠胚胎成纤维细胞的抑制浓度值为50%,比结合物对MCF
表征谱图
-
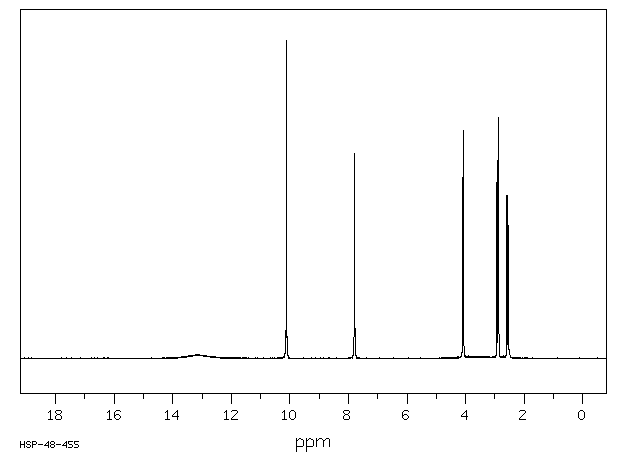
氢谱1HNMR
-
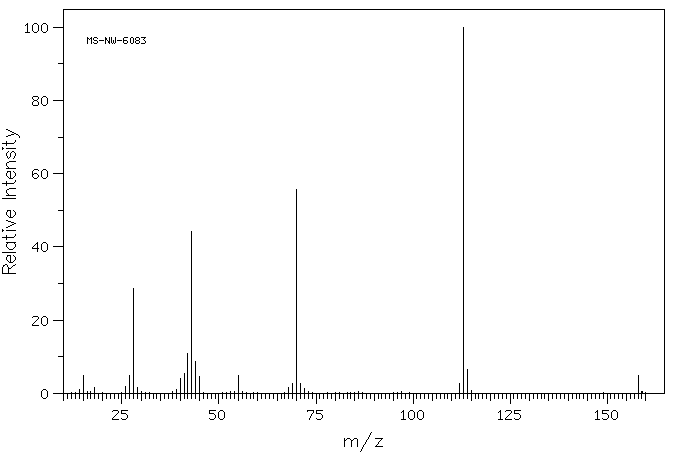
质谱MS
-
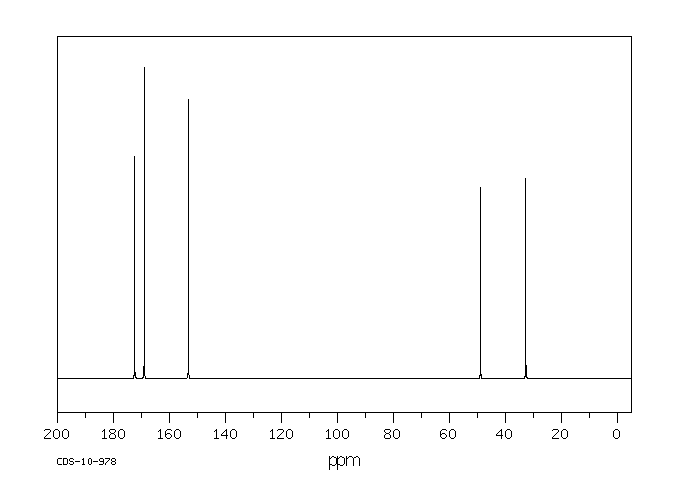
碳谱13CNMR
-
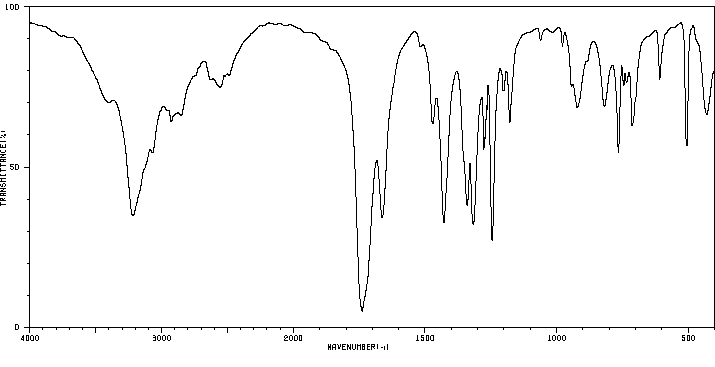
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸