N-炔丙基氧基酞亚胺 | 4616-63-1
中文名称
N-炔丙基氧基酞亚胺
中文别名
N-(炔丙基氧基)邻苯二甲酰亚胺
英文名称
2-(prop-2-yn-1-yloxy)isoindoline-1,3-dione
英文别名
N-propargyloxyphthalimide;2-(Prop-2-ynyloxy)-isoindole-1,3-dione;N-(Propargyloxy)phthalimide;2-prop-2-ynoxyisoindole-1,3-dione
CAS
4616-63-1
化学式
C11H7NO3
mdl
MFCD00005890
分子量
201.181
InChiKey
HBGZBVPXPDNXOV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:150-152 °C (lit.)
-
沸点:339.09°C (rough estimate)
-
密度:1.3041 (rough estimate)
-
溶解度:可溶于氯仿(少许)
-
稳定性/保质期:
常温常压下稳定,熔点:150-152°C。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:46.6
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2925190090
-
危险性防范说明:P264,P302+P352,P304+P340,P305+P351+P338,P332+P313,P337+P313
-
危险性描述:H315,H319,H335
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | N-(Propargyloxy)phthalimide 98% Material Safety Data Sheet |
| Synonym: | N-(Prop-2-ynyloxy)phthalimid |
| CAS: | 4616-63-1 |
Synonym:N-(Prop-2-ynyloxy)phthalimid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4616-63-1 | N-(Propargyloxy)phthalimide | 98 | 225-024-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4616-63-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige brown
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 149.00 - 150.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C11H7NO3
Molecular Weight: 201.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4616-63-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N-(Propargyloxy)phthalimide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 4616-63-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4616-63-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4616-63-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
功能性的炔烃
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-羟基邻苯二甲酰亚胺 N-hydroxyphthalimide 524-38-9 C8H5NO3 163.133 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-(4-Pyrrolidin-1-ylbut-2-ynoxy)isoindole-1,3-dione 74500-81-5 C16H16N2O3 284.315 —— 2-(4-Piperidin-1-ylbut-2-ynoxy)isoindole-1,3-dione 83108-14-9 C17H18N2O3 298.342 2-[4-(氮杂环辛烷-1-基)丁-2-炔氧基]异吲哚-1,3-二酮 2-(4-Azocan-1-yl-but-2-ynyloxy)-isoindole-1,3-dione 74484-70-1 C19H22N2O3 326.395 2-[4-(氮杂环庚-1-基)丁-2-炔氧基]异吲哚-1,3-二酮 2-((4-(Hexahydro-1H-azepin-1-yl)-2-butynyl)oxy)-1H-isoindole-1,3(2H)-dione 74484-69-8 C18H20N2O3 312.368 2-[4-(2-甲基哌啶-1-基)丁-2-炔氧基]异吲哚-1,3-二酮 2-((4-(2-Methyl-1-piperidinyl)-2-butynyl)oxy)-1H-isoindole-1,3(2H)-dione 74484-67-6 C18H20N2O3 312.368 —— 1H-Isoindole-1,3(2H)-dione, 2-((4-(2,6-dimethyl-1-piperidinyl)-2-butynyl)oxy)-, cis- 74484-68-7 C19H22N2O3 326.395
反应信息
-
作为反应物:描述:参考文献:名称:钯催化串联环化-阴离子捕获。第8部分:在固相上进行级联加氢苯乙烯基化-环化-阴离子捕获和级联加氢硼化-环化-阴离子捕获摘要:在五个组分过程(五点多样性)中,通过炔烃的硼氢化或加氢甲锡化利用树脂结合的芳基碘化物,然后进行涉及Suzuki或Stille反应的环化-阴离子捕获,可创建多达四个键和五个立体中心。准备了三个小型文库以验证化学性质。DOI:10.1016/s0040-4020(01)01061-4
-
作为产物:描述:参考文献:名称:[EN] NOVEL ORALLY BIOAVAILABLE BREATHING CONTROL MODULATING COMPOUNDS, AND METHODS OF USING SAME
[FR] NOUVEAUX COMPOSÉS DE MODULATION DE RÉGULATION DE LA RESPIRATION ORALEMENT BIODISPONIBLES ET LEURS PROCÉDÉS D'UTILISATION摘要:公开号:WO2014078575A3
文献信息
-
[EN] EXPANDED THERAPEUTIC POTENTIAL IN NITROHETEROARYL ANTIMICROBIALS<br/>[FR] POTENTIEL THÉRAPEUTIQUE ÉTENDU DANS DES ANTIMICROBIENS À NITROHÉTÉROARYLE申请人:UNIV CALIFORNIA公开号:WO2014205414A1公开(公告)日:2014-12-24Disclosed herein are antimicrobial compounds compositions, pharmaceutical compositions, the use and preparation thereof. Some embodiments relate to imidazole, thiazole, and furan derivatives and their use as therapeutic agents.
-
Nematicidal salicylaldehyde derivatives申请人:FMC Corporation公开号:US04584318A1公开(公告)日:1986-04-22A method is described for the control of nematodes in agricultural crops which comprises applying to the situs of infestation of a nematicidal composition containing as active ingredient a compound of the formula ##STR1## wherein Q is hydroxy, carbamoyloxy, or acyloxy, R is alkyl, alkenyl, alkynyl, X is halogen or methyl, n is 1 or 2. Preparation of active ingredient compounds is described, and nematicidal utility of compositions is exemplified.描述了一种用于控制农作物中线虫的方法,包括向线虫侵染部位施用一种含有如下活性成分的线虫杀剂组合物:其中Q为羟基、氨基甲酰氧基或酰氧基,R为烷基、烯基、炔基,X为卤素或甲基,n为1或2。描述了活性成分化合物的制备,并举例说明了组合物的线虫杀性效用。
-
Visible‐Light‐Mediated Click Chemistry for Highly Regioselective Azide–Alkyne Cycloaddition by a Photoredox Electron‐Transfer Strategy作者:Zheng‐Guang Wu、Xiang‐Ji Liao、Li Yuan、Yi Wang、You‐Xuan Zheng、Jing‐Lin Zuo、Yi PanDOI:10.1002/chem.202000252日期:2020.5.4Click chemistry focuses on the development of highly selective reactions using simple precursors for the exquisite synthesis of molecules. Undisputedly, the CuI -catalyzed azide-alkyne cycloaddition (CuAAC) is one of the most valuable examples of click chemistry, but it suffers from some limitations as it requires additional reducing agents and ligands as well as cytotoxic copper. Here, we demonstrateClick化学专注于使用简单的前体进行分子的精细合成的高选择性反应的开发。毋庸置疑,CuI催化的叠氮化物-炔烃环加成(CuAAC)是点击化学最有价值的例子之一,但由于需要额外的还原剂和配体以及细胞毒性铜,因此受到一些限制。在这里,我们展示了一种新颖的叠氮化物-炔烃环加成反应的策略,该策略涉及光氧化还原电子转移自由基机理,而不是传统的金属催化配位过程。这种新开发的光催化的叠氮化物-炔烃环加成反应可以在室温下,在空气和可见光存在的条件下,在温和条件下进行,显示出良好的官能团耐受性,优异的原子经济性,高达99%的高收率,和绝对的区域选择性,提供了各种1,4-二取代的1,2,3-三唑衍生物,包括生物活性分子和药物。使用可回收的光催化剂,太阳能和水作为溶剂,使该光催化系统具有可持续性和环境友好性。此外,叠氮化物-炔烃环加成反应可以在具有优异区域选择性的无金属催化剂的存在下进行光催化,这代表了点击化学
-
Click and Pick: Identification of Sialoside Analogues for Siglec-Based Cell Targeting作者:Cory D. Rillahan、Erik Schwartz、Ryan McBride、Valery V. Fokin、James C. PaulsonDOI:10.1002/anie.201205831日期:2012.10.29Click ‘n’ chips: Azide and alkyne‐bearing sialic acids (purple diamond; see picture) were subjected to high‐throughput click chemistry to generate a library of sialic acid analogues. Microarray printing of the library and screening with the siglec family of sialic‐acid‐binding proteins, led to the identification of high‐affinity ligands for siglec‐9 and siglec‐10.
-
Synthesis and antiviral evaluation of 4′-(1,2,3-triazol-1-yl)thymidines作者:Sanjeev Kumar V. Vernekar、Li Qiu、Jeana Zacharias、Robert J. Geraghty、Zhengqiang WangDOI:10.1039/c4md00039k日期:——novel antiviral nucleoside scaffolds, we have explored azide–alkyne cycloaddition reactions with 4′-azidothymidine (ADRT). The Ru-mediated reaction failed and the Cu-catalyzed variant generated 1,2,3-triazoles (9a–y) with only modest yields and efficiencies, indicating a substantial steric barrier around the 4′-azido group. Antiviral screening identified a few triazole analogues moderately active against在4'位置带有线性取代基(叠氮基或乙炔基)的非专性链终止核苷代表了抗病毒发现中一类重要的化合物,特别是针对丙型肝炎病毒(HCV)和人类免疫缺陷病毒(HIV)的化合物。先前我们已经证明3'-叠氮胸苷(AZT)衍生的1,2,3-三唑可能是HIV-1的有效抑制剂。为了评估功能化4'-线性取代基并可能产生新的抗病毒核苷支架的药物化学影响,我们探索了4'-叠氮胸苷(ADRT)的叠氮化物-炔烃环加成反应。Ru介导的反应失败,Cu催化的变异体生成1,2,3-三唑(9a–y)仅具有适度的产量和效率,表明4'-叠氮基团周围存在明显的空间位阻。抗病毒筛选确定了一些三唑类似物,对HIV-1(对10μM的抑制率为18–62%)和/或甲型流感病毒(对10μM的抑制率为15–50%)具有中等活性,对西尼罗河病毒(WNV)没有活性或HCV。这些结果表明,ADRT的线性4'叠氮基可能对靶标结合必不可少,并且其化学操作可能会大大削弱抗病毒效力。
表征谱图
-
氢谱1HNMR
-
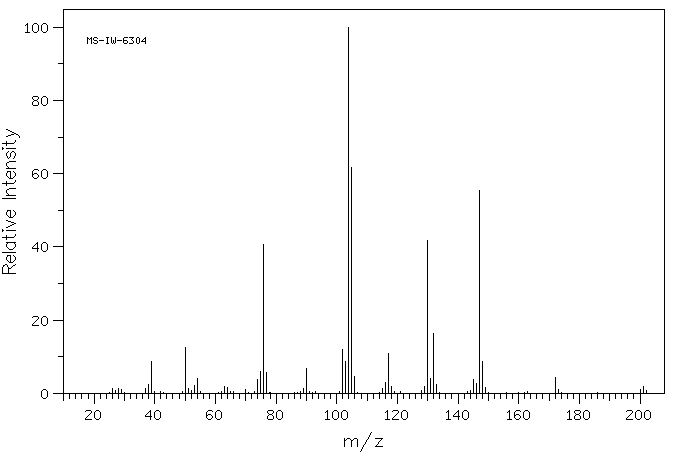
质谱MS
-
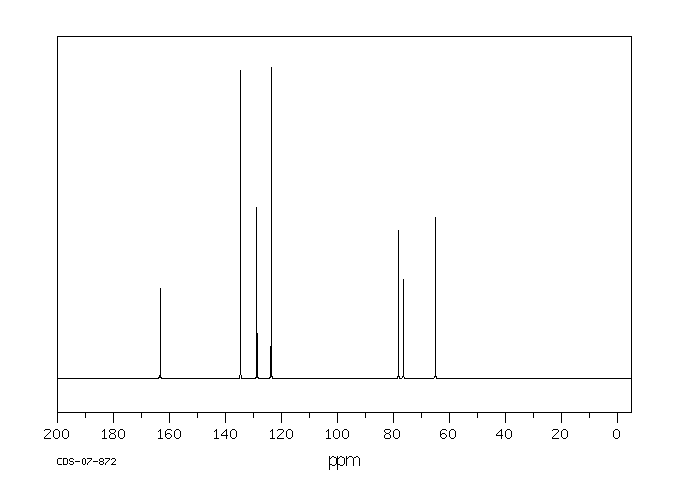
碳谱13CNMR
-
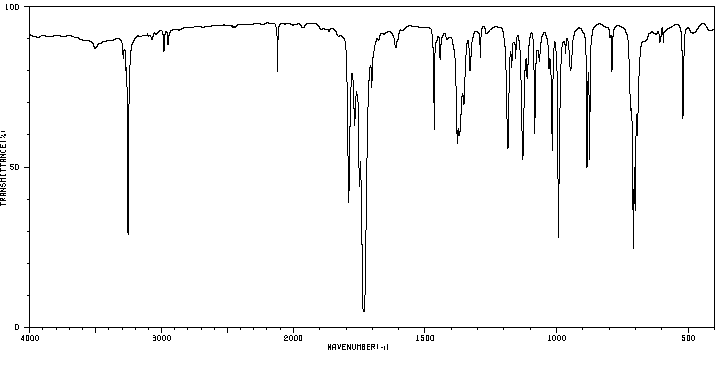
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z,3Z)-1,3-双[[((4S)-4,5-二氢-4-苯基-2-恶唑基]亚甲基]-2,3-二氢-5,6-二甲基-1H-异吲哚
鲁拉西酮杂质33
鲁拉西酮杂质07
马吲哚
颜料黄110
顺式-六氢异吲哚盐酸盐
顺式-2-[(1,3-二氢-1,3-二氧代-2H-异吲哚-2-基)甲基]-N-乙基-1-苯基环丙烷甲酰胺
顺式-2,3,3a,4,7,7a-六氢-1H-异吲哚
顺-N-(4-氯丁烯基)邻苯二甲酰亚胺
降莰烷-2,3-二甲酰亚胺
降冰片烯-2,3-二羧基亚胺基对硝基苄基碳酸酯
降冰片烯-2,3-二羧基亚胺基叔丁基碳酸酯
阿胍诺定
阿普斯特降解杂质
阿普斯特杂质FA
阿普斯特杂质68
阿普斯特杂质29
阿普斯特杂质27
阿普斯特杂质26
阿普斯特杂质19
阿普斯特杂质08
阿普斯特杂质03
阿普斯特杂质
阿普斯特二聚体杂质
阿普斯特
防焦剂MTP
铝酞菁
铁(II)1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-十六氟-29H,31H-酞菁
铁(II)2,9,16,23-四氨基酞菁
钠S-(2-{[2-(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙基]氨基}乙基)氢硫代磷酸酯
酞酰亚胺-15N钾盐
酞菁锡
酞菁二氯化硅
酞菁 单氯化镓(III) 盐
酞美普林
邻苯二甲酸亚胺
邻苯二甲酰基氨氯地平
邻苯二甲酰亚胺,N-((吗啉)甲基)
邻苯二甲酰亚胺阴离子
邻苯二甲酰亚胺钾盐
邻苯二甲酰亚胺钠盐
邻苯二甲酰亚胺观盐
邻苯二亚胺甲基磷酸二乙酯
那伏莫德
过氧化氢,2,5-二氢-5-苯基-3H-咪唑并[2,1-a]异吲哚-5-基
达格吡酮
诺非卡尼
螺[环丙烷-1,1'-异二氢吲哚]-3'-酮
螺[异吲哚啉-1,4'-哌啶]-3-酮盐酸盐
葡聚糖凝胶G-25