2-丙炔-1-醇 | 107-19-7
中文名称
2-丙炔-1-醇
中文别名
丙炔醇;3-羟基丙炔;炔丙醇;羟甲基乙炔;乙炔基甲醇
英文名称
propargyl alcohol
英文别名
Prop-2-ynyl alcohol;propargylic alcohol;propargyl acohol;2-propyn-1-ol;prop-2-yn-1-ol;2-propynol;2-propynyl alcohol;2-propyne-1-ol;propynyl alcohol
CAS
107-19-7
化学式
C3H4O
mdl
MFCD00002912
分子量
56.0642
InChiKey
TVDSBUOJIPERQY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
- 避免受热,避免与强氧化剂、强酸、强碱、酰基氯和酸酐接触。
- 有毒。对皮肤和眼睛有严重刺激作用。大鼠口服LD50为0.07g/kg。操作时应戴防护眼镜及手套。
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
ADMET
代谢
... 虽然低分子量一级醇的氧化代谢通常是由醇脱氢酶催化的,但是炔丙醇是这种酶的一个相对较差的底物 ... 研究了过氧化氢酶替代途径。使用了牛肝过氧化氢酶来测量炔丙醇氧化生物活化为2-丙炔-1-醛的速率 ... 发现这个速率比预测的要高 ... 并假设肝脏过氧化氢酶将炔丙醇氧化生物转化为更具反应性的α, β-不饱和醛可能是炔丙醇诱导的肝损伤的初始步骤。
... While oxidative metabolism of low molecular weight primary alcohols is generally ... catalyzed by alcohol dehydrogenase, propargyl alcohol is a relatively poor substrate for this enzyme ... The catalase alternative pathway /was studied/. Bovine liver catalase was used, to measure the rate of oxidative bioactivation of propargyl alcohol to 2-propyn-l-al ... /It was/ found the rate to be higher than predicted ... and hypothesized that the oxidative biotransformation of propargyl alcohol to the more reactive alpha, beta-unsaturated aldehyde by liver catalase might be the initial step in propargyl alcohol induced liver injury.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Chromatographic analysis indicated that propargyl alcohol is extensively metabolized and one metabolite was identified as a glutathione conjugate. It was assumed that there are multiple glutathione conjugates across the triple bond ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
在分离的肝细胞中,过氧化氢酶的失活仅部分抑制了炔丙醇的毒性。研究表明,炔丙醇诱导的细胞毒性、迅速的谷胱甘肽(GSH)耗竭和活性氧种(ROS)的形成涉及细胞色素P450的代谢激活,而不是过氧化氢酶或醇脱氢酶。通过特定的诱导和耗竭实验,证明了CYP 2E1是负责将炔丙醇激活为其醛,2-丙炔-1-醛的酶。提出了一个模型,即炔丙醇主要通过CYP 2E1氧化,醇脱氢酶也有少量贡献,生成2-丙炔-1-醛。这个醛是一种化学活性物质,可以攻击关键的细胞大分子,但它更倾向于与谷胱甘肽反应形成结合物,通过尿液排出。醛的另一种途径是通过醛脱氢酶进一步酶促氧化为丙烯酸,丙烯酸可以进一步氧化、结合并排出,或者再次转化为醛。谷胱甘肽的耗竭和ROS的形成支持了所提出的机制。
... Inactivation of catalase in isolated hepatocytes only partially inhibited the toxicity of propargyl alcohol /was shown/ ... Propargyl alcohol-induced cytotoxicity, rapid GSH depletion and reactive oxygen species (ROS) formation involves metabolic activation by cytochrome P450 rather than catalase or alcohol dehydrogenase /was demonstrated/. Using specific induction and depletion ... /it was shown/ that CYP 2E1 was the enzyme responsible for activation of propargyl alcohol to its aldehyde, 2-propyn-I-al ... /A model was proposed that/ propargyl alcohol is oxidized primarily by CYP 2E1, with a minor contribution by alcohol dehydrogenase, to 2-propyn-l-al. The aldehyde is a chemically active species that can attack vital cellular macromolecules but reacts preferentially with glutathione to form conjugates that undergo urinary excretion. An alternative pathway for the aldehyde is further enzymatic oxidation via aldehyde dehydrogenase to propiolic acid, which could be further, oxidized, conjugated and excreted, or be converted back into the aldehyde ... Depletion of glutathione and formation of ROS (reactive oxygen species), which lends support to the proposed mechanism, /was demonstrated/ ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
Sprague-Dawley雄性大鼠通过口服给予40 mg/kg的(1,2,3-(13)C)炔丙醇和(1,2-(14)C)炔丙醇混合物。大约60%的剂量在96小时内通过尿液排出。通过1-和2-D NMR在尿液中鉴定出主要代谢物,并通过分离和纯化各个代谢物,随后使用(13)C FT-NMR和质谱法进行确认。提出的代谢途径包括炔丙醇氧化成2-炔酸和谷胱甘肽结合,这是首次发现多个谷胱甘肽加成到三键上的例子。确定的最终产物包括:3-((2-(乙酰氨基)-2-羧乙基)硫基)-2-丙烯酸,S-S-(3-羟基丙基亚基)-双(N-乙酰半胱氨酸),以及3-((2-(乙酰氨基)-2-羧乙基)亚磺酰基)-3-((2-(乙酰氨基)-2-羧乙基)硫基)-1-丙醇。
Male Sprague-Dawley rats were dosed orally with 40 mg/kg mixture of (1,2,3-(13)C)propargyl alcohol and (1,2-(14)C)propargyl alcohol. Approximately 60% of the dose was excreted in the urine by 96 hr. Major metabolites were identified in the urine by 1- and 2-D NMR and confirmed by isolation and purification of the individual metabolites followed by (13)C FT-NMR and mass spectometry. The proposed pathway involves oxidation of propargyl alcohol to 2-propynoic acid and glutathione conjugation, the first example of multiple glutathione additions to a triple bond. The following final products were identified: 3-((2-(acetylamino)-2-carboxyethyl)thio)-2-propenoic acid, S-S-(3-hydroxypropylidene)-bis(N-acetylcysteine), and 3-((2-(acetylamino)-2-carboxyethyl)-sulfinyl)-3-((2-(acetylamino)-2-carboxyethyl)thio)1-propanol.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
该物质可以通过吸入其蒸汽、通过皮肤接触以及摄入进入人体。
The substance can be absorbed into the body by inhalation of its vapour, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,皮肤吸收,吞食,皮肤和/或眼睛接触
inhalation, skin absorption, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
皮肤刺激,粘膜;中枢神经系统抑制;在动物中:肝脏,肾脏损害
irritation skin, mucous membrane; central nervous system depression; In Animals: liver, kidney damage
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
咳嗽。喉咙痛。
Cough. Sore throat.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
可能被吸收!红色。
MAY BE ABSORBED! Redness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
丙烯醇在静脉注射给药后迅速分布并排泄。大部分放射性(标记的测试材料)通过大鼠和小鼠的尿液和呼吸中的二氧化碳排出。口服给药后,也出现了类似的快速(但比静脉给药后慢)排泄模式,大部分放射性通过尿液排出和呼出的二氧化碳。由于丙烯醇的挥发性,经皮吸收率较低。吸入暴露导致在1或10 ppm时吸入丙烯醇的吸收率为55至63%,而在100 ppm时仅为23至33%。两种物种主要通过尿液排出了吸入剂量的多数。
Propargyl alcohol is quickly distributed and excreted following an iv dose. The majority of the radioactivity ((14)C-labeled test material) was excreted in the urine and as carbon dioxide in the breath of both rats and mice. Oral dosing resulted in a similar rapid (but slower than after iv dosing) excretion pattern, with the bulk of radioactivity being excreted in the urine and exhaled carbon dioxide. Dermal absorption was low due to the volatility of propargyl alcohol. Inhalation exposure resulted in 55 to 63% absorption of inhaled propargyl alcohol at 1 or 10 ppm and only 23 to 33% absorption at 100 ppm. Both species eliminated the majority of the inhaled dose in urine.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
丙烯醇可以通过吸入其蒸汽、透过皮肤和摄入的方式被人体吸收。
... /Propargyl alcohol/ can be absorbed into the body by inhalation of its vapor, through the skin and by ingestion.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Male Sprague-Dawley rats were dosed orally with 40 mg/kg mixture of (1,2,3-(13)C)propargyl alcohol and (1,2-(14)C)propargyl alcohol. Approximately 60% of the dose was excreted in the urine by 96 hr ...
来源:Hazardous Substances Data Bank (HSDB)
制备方法与用途
制备方法
其制备方法是由乙炔和甲醛经催化加成而得。反应在串联的管式反应器中进行,在1.96MPa的压力下,将乙炔加入5%~6%甲醛水溶液中,该溶液悬浮着粉状乙炔铜催化剂。维持反应温度在100~125℃之间,反应液通过气液分离器分离,过剩的乙炔循环使用。反应液经过压滤器除去乙炔铜催化剂后进行蒸馏,得到丙炔醇水溶液。再经回收甲醇、脱水分馏而得丙炔醇,其含量在96%以上。蒸馏釜残留液为副产物1,4-丁炔二醇,收率为丙炔醇37%,丁炔二醇50%,总收率87%。
在国外,有的采用四氢呋喃或二甲基甲酰胺作为溶剂,还有的采用气相反应(常压下以铬酸铜为催化剂),以减少丁炔二醇的生成。
合成制备方法其制备方法同样是由乙炔和甲醛经催化加成而得。反应在串联的管式反应器中进行,在1.96MPa的压力下,将乙炔加入5%~6%甲醛水溶液中,该溶液悬浮着粉状乙炔铜催化剂。维持反应温度在100~125℃之间,反应液通过气液分离器分离,过剩的乙炔循环使用。反应液经过压滤器除去乙炔铜催化剂后进行蒸馏,得到丙炔醇水溶液。再经回收甲醇、脱水分馏而得丙炔醇,其含量在96%以上。蒸馏釜残留液为副产物1,4-丁炔二醇,收率为丙炔醇37%,丁炔二醇50%,总收率87%。
在国外,有的采用四氢呋喃或二甲基甲酰胺作为溶剂,还有的采用气相反应(常压下以铬酸铜为催化剂),以减少丁炔二醇的生成。
用途简介丙炔醇是一种重要的有机合成中间体。在医药行业中,它是合成磷霉素钠、磷霉素钙和磺胺嘧啶的关键中间体。此外,它还是镀镍光亮剂,能产生光亮和整平效果。
用途上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丁炔二醇 1,4-dihydroxybut-2-yne 110-65-6 C4H6O2 86.0904 —— 3-ethoxy-1-propyne 628-33-1 C5H8O 84.1179 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-丁炔-1-醇 methyl propargyl alcohol 764-01-2 C4H6O 70.091 甲基炔丙基醚 Methyl propargyl ether 627-41-8 C4H6O 70.091 丁炔二醇 1,4-dihydroxybut-2-yne 110-65-6 C4H6O2 86.0904 3-碘-2-丙炔醇 3-iodo-2-propyn-1-ol 1725-82-2 C3H3IO 181.961 1-溴丙炔-3-醇 3-bromoprop-2-yn-1-ol 2060-25-5 C3H3BrO 134.96 —— 3-chloro-2-propyn-1-ol 38987-68-7 C3H3ClO 90.5092 —— 3-ethoxy-1-propyne 628-33-1 C5H8O 84.1179 —— 2,4-pentadiyne-1-ol 41345-53-3 C5H4O 80.0862
反应信息
-
作为反应物:描述:参考文献:名称:Complete kinetic analysis of thermal stereomutations among the eight 2,3-dideuterio-2-(methoxymethyl)spiro[cyclopropane-1,1'-indenes]摘要:DOI:10.1021/ja00316a036
-
作为产物:描述:参考文献:名称:Acetylenic compositions and nickel plating baths containing same摘要:本文介绍了一种新的乙炔基化合物,3-(2-丙炔氧基)-2-丙烯酸及其制备方法。同时,本文还介绍了一种制备乙炔基化合物的方法,该方法包括以下步骤:(a)在水性碱性溶液中反应丙炔醇和碱金属高锰酸盐,(b)过滤反应混合物,(c)用酸使过滤液酸化。用这种方法制备的乙炔基化合物可用于水性酸性电镀浴中,在基材上电沉积出明亮的镍或镍铁合金。本文还介绍了从这种电镀浴中电沉积镍和镍铁合金的方法,以及形成电镀浴的添加剂组成。公开号:US04421611A1
-
作为试剂:参考文献:名称:Ru(II)-催化的烯丙基化和 N-甲苯磺酰苯甲酰胺与炔丙醇的连续环化摘要:我们在此报告了 Ru( II ) 催化的N-甲苯磺酰苯甲酰胺的C(sp 2 )–H 烯丙基化以获得多取代的烯丙基酰胺。此外,通过碱介导的环化将丙二烯基酰胺转化为相应的异喹诺酮衍生物。目前的协议具有催化剂负载低、反应条件温和、官能团兼容性高和所需的可扩展性等特点。所提供的丙二烯的独特功能允许进一步转换以扩展协议的实用性。DOI:10.1039/d1cc01768c
文献信息
-
Some Aspects of the Azide-Alkyne 1,3-Dipolar Cycloaddition Reaction作者:N. T. Pokhodylo、M. A. Tupychak、O. Ya. Shyyka、M. D. ObushakDOI:10.1134/s1070428019090082日期:2019.9Some peculiar features of two most commonly used catalytic systems (Cul and CuSOVsodium ascorbate) controlling the regioselectivity of 1,3-dipolar cycloaddition of azides to terminal alkynes have been studied. Their potentialities, main disadvantages, and limitations have been demonstrated by a number of examples, including reactions of low-molecular-weight azides and alkynes containing heterocyclic
-
一种用于治疗肿瘤的黄酮衍生物及其应用申请人:四川福生源科技有限公司公开号:CN111620920A公开(公告)日:2020-09-04
-
Enantioselective Rhodium-Catalyzed Atom-Economical Macrolactonization作者:Stephanie Ganss、Bernhard BreitDOI:10.1002/anie.201604301日期:2016.8.8coupling of ω‐allenyl‐substituted carboxylic acids. The use of a modified diop ligand, chiral DTBM‐diop, led to high enantioselectivity (up to 93 % ee). The reaction tolerated a large variety of functionalities, including α,β‐unsaturated carboxylic acids and depsipeptides, and provided the desired macrocycles with very high enantio‐ and diastereoselectivity.
-
Synthesis, biological activities and structure−activity relationships for new avermectin analogues作者:Jian Zhang、Xiang Nan、Hai-Tao Yu、Pi-Le Cheng、Yan Zhang、Ying-Qian Liu、Shao-Yong Zhang、Guan-Fang Hu、Huanxiang Liu、An-Liang ChenDOI:10.1016/j.ejmech.2016.05.056日期:2016.10LC50 values of 7.744, 5.634, 6.809, 7.939 and 52.234 μM, respectively. Furthermore, QSAR analysis showed that the molecular shape, size, connectivity degree and electronic distribution of avermectin analogues had substantial effects on insecticidal potency. These preliminary results provided useful insight in guiding further modifications of avermectin in the development of potential new insecticides.为了发现具有良好杀虫活性的新分子,合成了40多种新的阿维菌素衍生物,并评估了它们对三种物种的蜘蛛,昆虫和线虫的生物活性,这些物种分别是朱砂叶螨(Tetranychus Cinnabarinus),蚜虫(Aphis craccivora)和松柏xylophilus。所有测试的化合物均显示出对三种昆虫的有效抑制活性。值得注意的是,大多数化合物对朱砂毛虫表现出高选择性,其中一些与阿维菌素相比具有更好的选择性。特别是化合物9j(LC 50:0.005μM)和16d(LC 50:0.002μM)对朱砂丁香的活性分别比阿维菌素(LC 50: 0.013μM )高2.5倍和4.7倍。此外,化合物9B,9D-F ,9H,9J,9升,9N,9P,9R,9V和17D显示了优良的活性与LC 50个相比的2.959-5.013μM的值1(LC 50: 6.746μM)对乙。松材线虫。同时,化合物9f,9g,9h和9m的杀虫活性针对A
-
A new synthesis of phthalimidines作者:L. Yu. Ukhin、K. Yu. Suponitsky、L. V. Belousova、Zh. I. OrlovaDOI:10.1007/s11172-009-0347-1日期:2009.12A new synthesis of phthalimidines is described. 3-Acyloxy-2-aryl- and 2-acylamino-3-acyloxyphthalimidines were prepared by the reaction of 3-arylaminophthalides or o-formylbenzoic acid acylhydrazones with acetic or propionic anhydrides. Their reactions with O-, N-, S-, and C-nucleophiles were studied. The structure of 2-acetyl(cyanoacetyl)amino-3-acetoxyindolin-1-one was confirmed by X-ray diffraction
表征谱图
-
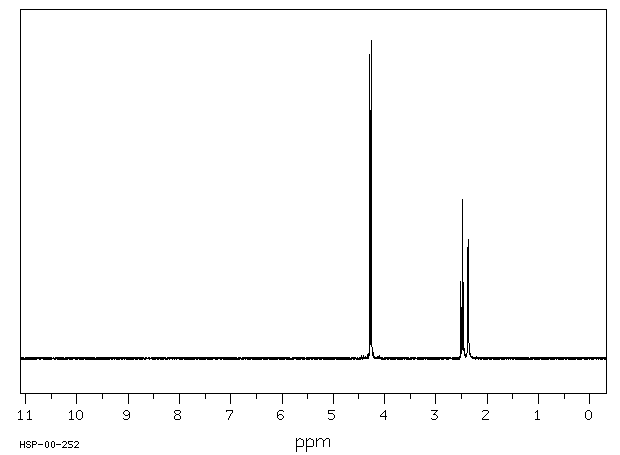
氢谱1HNMR
-
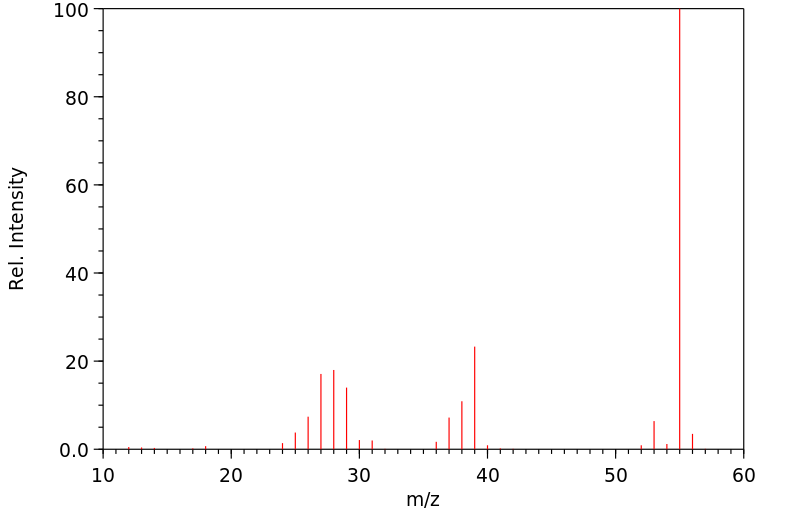
质谱MS
-
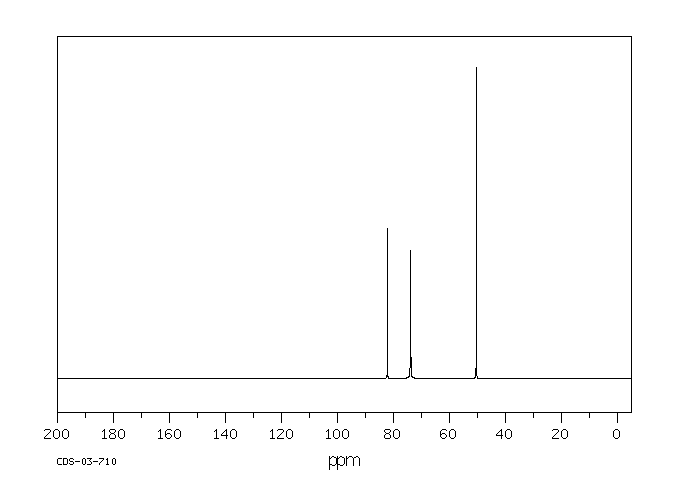
碳谱13CNMR
-
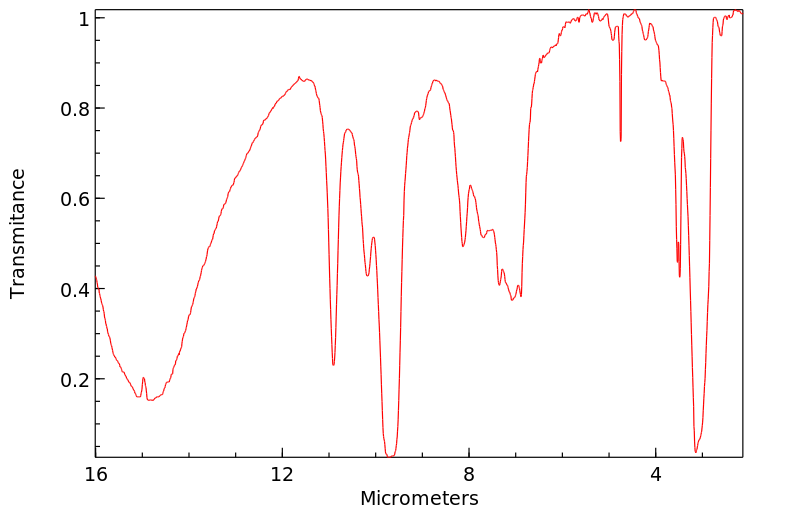
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锗烷,三甲基[3-(三甲基甲锡烷基)-2-炔丙基]-
锗烷,三甲基-2-炔丙基-
铜,1-戊炔基-
甲基炔丙基硫化物
甲基乙炔和丙二烯混合物
甲基丙-2-炔基氰基二硫代亚氨酸酯
甲基-D3-乙炔
环戊基乙炔
环己基乙炔
环丙乙炔
炔丙胺
炔丙基膦
炔丙基碘化物
炔丙基叔丁基二甲基硅烷
炔丙基三甲基硅烷
炔丙基三乙基硅烷
氘乙炔
戊-1-炔-3-胺
戊-1,3-二炔
戊-1,2-二烯-4-炔
异氰基-乙炔
己基(己-5-炔基)甲基硅烷
己-1-炔银
四碳化铀
反式-4-(2-丙炔基)-环己烷甲醇
双(三甲基锡)乙炔
双(三氟甲基)锌
十四碳-1,4-二炔
十四碳-1,3-二炔
十八碳-1,17-二炔
十八炔
十三碳-1,7-二炔
十三碳-1,12-二炔
十一碳-1,5-二炔
亚硫酸二(2-丙炔基)酯
二甲基炔丙基溴化硫
二炔丙基硫醚
二乙炔基-二甲基-锗烷
二丙-1-炔基汞
二[2-甲氧基乙基汞(II)]乙炔
二(三正丁基甲锡烷基)乙炔
二(3-羟基-1-丙炔基)汞(II)
乙炔锂乙二胺配合物
乙炔银
乙炔基环己烷钠
乙炔基环丙烷氯化镁
乙炔基(三甲基)锗烷
乙炔基(三甲基)硅烷铜(1+)
乙炔基(三甲基)硅烷溴化镁
乙炔基(三甲基)硅烷氯化镁