3-allyl-1H-indole-2-carboxylic acid | 908105-30-6
中文名称
——
中文别名
——
英文名称
3-allyl-1H-indole-2-carboxylic acid
英文别名
3-Allyl-2-indolecarboxylic acid;3-prop-2-enyl-1H-indole-2-carboxylic acid
CAS
908105-30-6
化学式
C12H11NO2
mdl
——
分子量
201.225
InChiKey
MFWJRIIPEPIWOE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:15
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:53.1
-
氢给体数:2
-
氢受体数:2
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-hydroxy-3,4,5,10-tetrahydro-2H-azepino[3,4-b]indol-1-one 1620658-67-4 C12H12N2O2 216.239
反应信息
-
作为反应物:描述:3-allyl-1H-indole-2-carboxylic acid 在 lithium hydroxide monohydrate 、 sodium hydride 作用下, 以 四氢呋喃 、 甲醇 、 水 、 N,N-二甲基甲酰胺 、 mineral oil 为溶剂, 反应 2.0h, 生成 3-allyl-1-methylindole-2-carboxylic acid参考文献:名称:Synthesis and biological evaluation of tetrahydro[1,4]diazepino[1,2-a]indol-1-ones as cyclin-dependent kinase inhibitors摘要:New series of 2,3,4,5-tetrahydro[1,4]diazepino[1,2-a]indol-1-ones and 3,4,5,10-tetrahydro-2H-diazepino[3,4-b]indol-1-ones have been synthesized through an iodolactonisation/lactone-to-lactam rearrangement sequence. These compounds were evaluated as potential protein kinase inhibitors (CDK1, CDK5 and GSK-3). 11-Iodo-2,3,4,5-tetrahydro[1,4]diazepino[1,2-a]indol-1-one derivatives exhibited sub-micromolar inhibitory activity against cyclin-dependent kinases. Docking studies were realized to determine the binding mode of the inhibitors into the ATP binding domain of the CDK5 catalytic site. Our result highlighted two weak Van-der-Waals bonding interactions established between the iodine atom and both phenyl group of Phe 80 and ammonium end of Lys 33.DOI:10.1016/j.ejmech.2014.06.063
-
作为产物:描述:1-tert-butyl 2-ethyl-3-iodo-1H-indole-1,2-dicarboxylate 在 四(三苯基膦)钯 、 sodium hydroxide 作用下, 以 乙醇 、 水 、 甲苯 为溶剂, 反应 24.0h, 生成 3-allyl-1H-indole-2-carboxylic acid参考文献:名称:Synthesis and biological evaluation of tetrahydro[1,4]diazepino[1,2-a]indol-1-ones as cyclin-dependent kinase inhibitors摘要:New series of 2,3,4,5-tetrahydro[1,4]diazepino[1,2-a]indol-1-ones and 3,4,5,10-tetrahydro-2H-diazepino[3,4-b]indol-1-ones have been synthesized through an iodolactonisation/lactone-to-lactam rearrangement sequence. These compounds were evaluated as potential protein kinase inhibitors (CDK1, CDK5 and GSK-3). 11-Iodo-2,3,4,5-tetrahydro[1,4]diazepino[1,2-a]indol-1-one derivatives exhibited sub-micromolar inhibitory activity against cyclin-dependent kinases. Docking studies were realized to determine the binding mode of the inhibitors into the ATP binding domain of the CDK5 catalytic site. Our result highlighted two weak Van-der-Waals bonding interactions established between the iodine atom and both phenyl group of Phe 80 and ammonium end of Lys 33.DOI:10.1016/j.ejmech.2014.06.063
文献信息
-
Substituted indole-2-carboxylic acids as glucosyl transferase inhibitors申请人:Pfizer Products Inc.公开号:EP0894789A1公开(公告)日:1999-02-035-Carbonylaryl, 5-carbonylalkyl and 5-carbonylcycloalkyl-3-alkyl-indole-2-carboxylic acids of formula (I) are useful as UGGTASE inhibitors. wherein: R1 is hydrogen or (C1-C6)alkyl; and R2 is (C1-C6)alkyl, (C3-C7)cycloalkyl, phenyl or phenyl substituted with one to three groups independently selected from (C1-C3)alkyl, (C1-C3)alkoxy, halogen, cyano or nitro; or a pharmaceutically acceptable salt thereof.
-
JPH11100367A申请人:——公开号:JPH11100367A公开(公告)日:1999-04-13
-
US5981762A申请人:——公开号:US5981762A公开(公告)日:1999-11-09
表征谱图
-
氢谱1HNMR
-
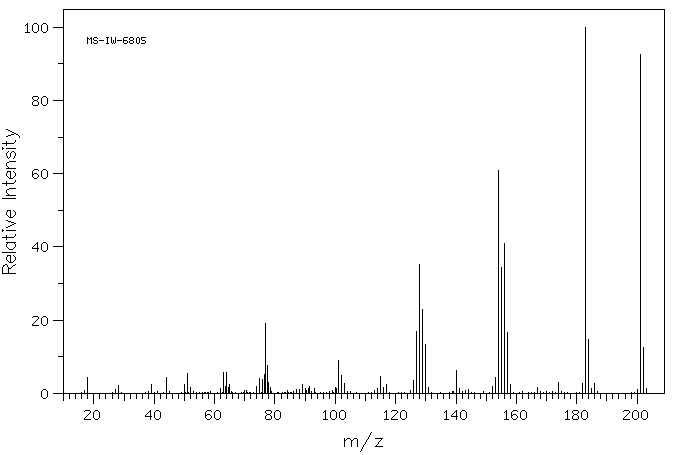
质谱MS
-
碳谱13CNMR
-
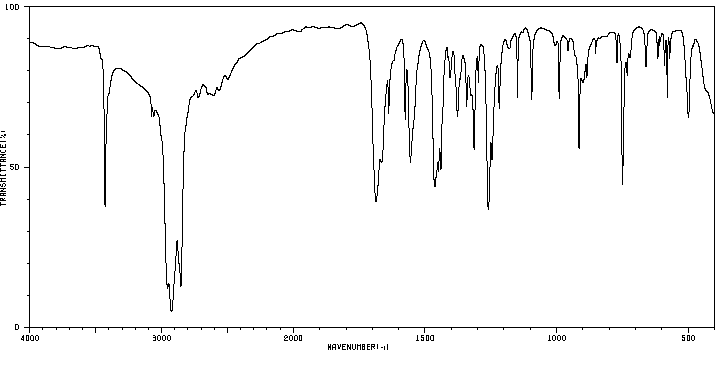
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3