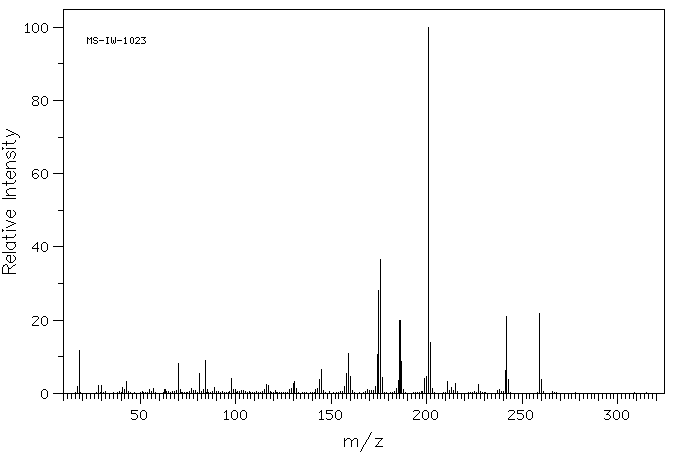
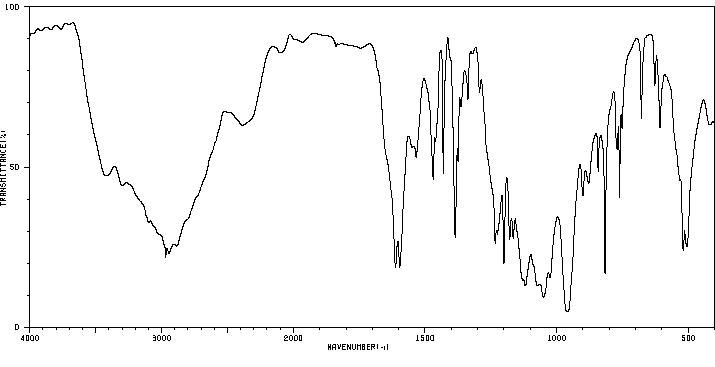
毒理性
◉ 母乳喂养期间使用总结:伯氨喹在哺乳期母亲的乳汁中排泄不良,在其母乳喂养的婴儿血清中检测不到。超过新生儿期的母乳喂养婴儿没有表现出溶血的证据。对于葡萄糖-6-磷酸脱氢酶(G6PD)缺乏的新生儿和婴儿尚未进行研究,然而,超过28天龄的G6PD缺乏婴儿从母乳中暴露似乎有较低的溶血风险。如果需要伯氨喹,建议在给哺乳期母亲用药前对母亲和婴儿进行G6PD缺乏的检测。
英国疟疾治疗指南建议,患有疟疾的哺乳期母亲应避免使用伯氨喹,并在停止哺乳前每周给予氯喹500毫克。然而,这些指南是在关于伯氨喹排入母乳和母乳喂养婴儿安全性的信息发布之前制定的。更新的信息表明,所有哺乳超过28天龄婴儿的母亲可以安全地接受伯氨喹。疾病控制与预防中心的指南指出,伯氨喹可以在G6PD水平正常的哺乳期母亲和婴儿中使用。由于母乳中转移的少量伯氨喹不足以提供足够的疟疾防护或治疗,需要化学预防或治疗的婴儿必须接受推荐剂量的伯氨喹。
◉ 对母乳喂养婴儿的影响:21位患有间日疟的母亲在哺乳期给予伯氨喹0.5毫克/千克/天,连续14天,她们的婴儿至少28天龄。在任何婴儿中都没有看到血细胞比容、海因茨小体计数、血清胆红素、氧饱和度或高铁血红蛋白血症的变化。
一位产后5个月患有间日疟的妇女给予伯氨喹0.52毫克/千克/天,连续7天,然后在重新检查患者体重后以0.46毫克/千克/天连续7天。她被发现是葡萄糖-6-磷酸脱氢酶(G6PD)缺乏的杂合子,并经历了溶血和贫血。她的女性婴儿在治疗期间正在哺乳(程度未说明),被发现是G6PD Mahidol变异的杂合子,但没有明显的溶血。孩子的疫苗接种计划已完成,6个月大的运动里程碑正常。
◉ 对泌乳和母乳的影响:截至修订日期,未找到相关已发布信息。
◉ Summary of Use during Lactation:Primaquine is poorly excreted into breastmilk of nursing mothers and undetectable in the serum of their breastfed infants. Breastfed infants beyond the neonatal period have shown no evidence of hemolysis. Neonates and infants with glucose-6-phosphate dehydrogenase (G6PD) deficiency have not been studied, however, G6PD-deficient infants over 28 days of age appear to have a low risk of hemolysis from exposure in breastmilk. If primaquine is required, testing the mother and infant for G6PD deficiency is advisable before the drug is given to a nursing mother.
United Kingdom malaria treatment guidelines recommend that primaquine be avoided in nursing mothers with malaria and that weekly chloroquine 500 mg be given until breastfeeding is completed. However, these guidelines were developed before information on the excretion of primaquine into breastmilk and safety in breastfed infants was published. More recent information indicates that all mothers nursing infant over 28 days of age could safely receive primaquine. The Centers for Disease Control and Prevention guidelines state that primaquine may be used in breastfeeding mothers and infants with normal G6PD levels. Because the small amounts of primaquine transferred in breast milk are insufficient to provide adequate protection or treatment of malaria, infants who require chemoprophylaxis or therapy must receive the recommended dosages of primaquine.
◉ Effects in Breastfed Infants:Twenty-one mothers with vivax malaria were give a dosage of primaquine 0.5 mg/kg daily for 14 days while breastfeeding their infants who were at least 28 days old. No alterations in hematocrit, Heinz body counts, serum bilirubin, oxygen saturation, or methemoglobinemia were seen in any of the infants.
A woman with vivax malaria who was 5 months postpartum was given a dose of primaquine of 0.52 mg/kg daily for 7 days, then 0.46 mg/kg daily for 7 days after rechecking the patient’s weight. Shwas found to be heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency and experienced some hemolysis and anemia. Her female infant was being breastfed (extent not stated) during treatment and was found to be heterozygous for the G6PD Mahidol variant, but had no apparent hemolysis. The child’s vaccination schedule was completed, and the 6-month motor milestones were normal.
◉ Effects on Lactation and Breastmilk:Relevant published information was not found as of the revision date.
来源:Drugs and Lactation Database (LactMed)